Germ cell tumors
Intratubular germ cell neoplasia
Seminoma
Classic
With syncytiotrophoblastic cells
Spermatocytic seminoma (mention if there is sarcomatous component)
Embryonal carcinoma
Yolk sac tumor
Choriocarcinoma
Teratoma (specify if with a secondary malignant component and type)
Tumors with more than one histological type (specify percentage of individual components)
Sex cord/gonadal stromal tumors
Leydig cell tumor
Malignant Leydig cell tumor
Sertoli cell tumor
Large cell calcifying
Sclerosing
Intratubular large cell hyalinizing
Malignant Sertoli cell tumor
Granulosa cell tumor
Juvenile type
Adult type
Thecoma/fibroma group of tumors
Tumors containing germ cell and sex cord/gonadal stromal components
Gonadoblastoma
Unclassified
Miscellaneous tumors of the testis
Carcinoid tumor
Brenner tumor
Tumors of ovarian epithelial types
Others
Metastatic tumors to the testis
A small metacentric marker chromosome, identified as an isochromosome of the short arm of chromosome 12 [i(12p)], that results in excess DNA from this locus, or other forms of chromosome 12p amplification, have been reported in almost all GCTs analyzed, suggesting that this karyotypic abnormality is characteristic of the whole spectrum of the postpubertal germ cell tumors of the testis [5] .
Intratubular Germ Cell Neoplasia
The evolution of the concept of intratubular germ cell neoplasia (also known as testicular intraepithelial neoplasia or carcinoma in situ) indicates that most adult germ cell tumors of the testis evolve from a common neoplastic precursor lesion: intratubular germ cell neoplasia, unclassified type (IGCNU). At 5 years about 50 % of patients with a testicular biopsy positive for IGCNU have developed invasive germ cell tumors , and only a small fraction remain free of invasive tumors by 7 years.
Microscopically, IGCNU is characterized by seminiferous tubules showing decreased or absent spermatogenesis . The cells normally lining the tubules are replaced by basally located undifferentiated germ cells. The malignant germ cells have the appearance of seminoma cells with enlarged, polygonal nuclei, coarse chromatin, enlarged single or multiple nucleoli, clear cytoplasm, and distinct cell membranes (Fig. 35.1). IGCNU cells express PLAP, CD117, SALL4, podoplanin, and OCT3/4 (Fig. 35.2a, b) (see Chap. 40).
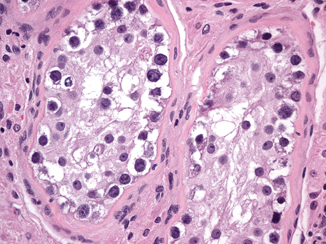
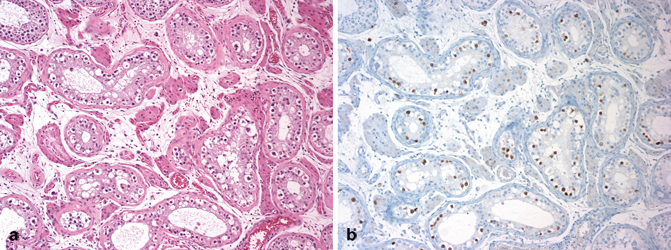

Fig. 35.1
Intratubular germ cell neoplasia, unclassified type (IGCNU). The malignant germ cells have enlarged, polygonal nuclei, coarse chromatin, enlarged single or multiple nucleoli, and clear cytoplasm (40X)

Fig. 35.2
The malignant cells of IGCNU (a) are positive for OCT3/4 (b) (10X)
Seminoma
Seminoma is the most frequent GCT (27–56 % of all germ cell neoplasms) and occurs most commonly in young to middle aged men (mean age at diagnosis 40 years) [6]. Grossly, it is typically a diffuse or multinodular soft tan-white mass (Fig. 35.3); focal necrosis may be present. Seminoma cells morphologically and immunophenotypically resemble embryonic germ cells. On microscopic examination, seminoma is characterized by a framework of delicate fibrous septa with associated blood vessels and a sprinkling of lymphocytes (Fig. 35.4). A granulomatous reaction, usually consisting of clusters of epithelioid histiocytes, is present in approximately 50 % of cases (Fig. 35.5). The tumor cells have distinct cytoplasmic membranes and polygonal nuclei, which often have “squared-off” nuclear edges, with large nucleoli (Fig. 35.6). Mitoses are variable in number but easily identified. In approximately 10 % of cases, syncytiotrophoblastic cells are identified on routine sections, but these cases are still classified as “pure” seminoma. Similar to IGCNU, seminoma cells express PLAP, CD117, SALL4, podoplanin, SOX17, and OCT3/4.
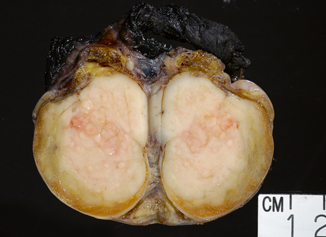
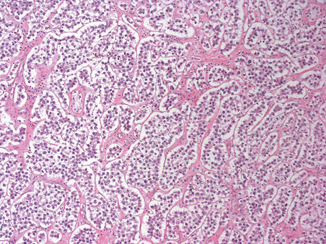
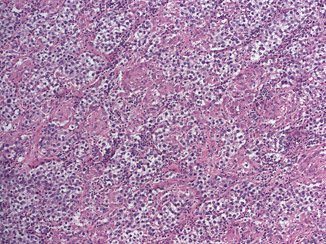
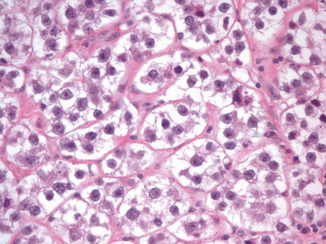

Fig. 35.3
Typical multinodular tan-white seminoma

Fig. 35.4
Seminoma composed of uniform cells divided into clusters by delicate fibrous septa associated with mild lymphocytic infiltrate (10X)

Fig. 35.5
Seminoma with pronounced granulomatous reaction (10X)

Fig. 35.6
Seminoma cells have distinct cytoplasmic membranes and polygonal nuclei, which often have “squared-off” nuclear edges, with large nucleoli (40X)
Seminoma, 80 % of which are diagnosed at stage I, is highly sensitive to both radiotherapy and chemotherapy and, therefore, cure is an expected outcome in the majority of cases, even with metastatic disease at presentation [7]. The classical and the syncytiotrophoblastic types of seminoma behave similarly, although the syncytiotrophoblastic subtype is associated with increased serum beta HCG levels. The spermatocytic type is infrequent, occurs in older men, and has a better prognosis since it rarely metastasizes (< 1 % of cases) in the absence of sarcomatous transformation, which is very rare. Spermatocytic seminoma and seminoma variants are discussed in Chap. 37.
Embryonal Carcinoma
Although pure embryonal carcinoma comprises approximately 10 % of testicular germ cell tumors , it occurs as a component in more than 80 % of mixed germ cell tumors, mostly in young men, with a peak of incidence around 30 years of age [4, 6] . Grossly, embryonal carcinoma is soft gray-red to tan-yellow with foci of hemorrhage and necrosis (Fig. 35.7). Microscopically it is composed of pleomorphic cells with abundant cytoplasm and large, irregular nuclei with prominent macronucleoli. The cells border are usually indistinct and the cells tend to crowd with overlapping nuclei (Fig. 35.8). Mitotic figures are frequent. The cells can form sheets or acinar, glandular (Fig. 35.9a), papillary (Fig. 35.9b), and tubular structures. Embryonal carcinomas express PLAP, OCT3/4, CD30, SALL4, SOX2, and cytokeratin; EMA and vimentin are usually negative.
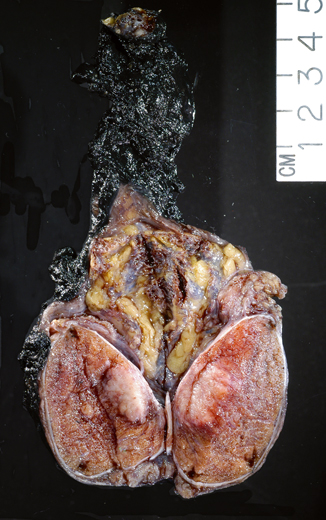
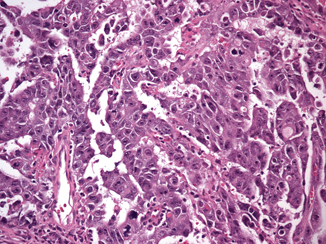
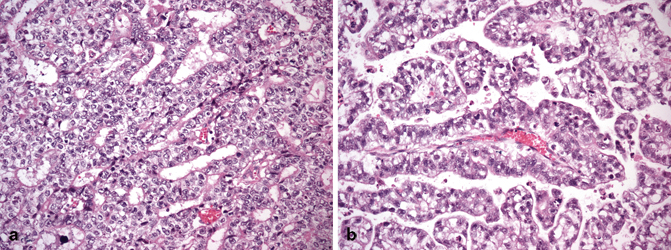

Fig. 35.7
Embryonal carcinoma grossly characterized by a soft gray-pink tumor with peripheral hemorrhage

Fig. 35.8
Embryonal carcinoma cells are pleomorphic with abundant cytoplasm and large, irregular nuclei with prominent macronucleoli. The cells border are usually indistinct and the cells tend to crowd with overlapping nuclei (20X)

Fig. 35.9
Embryonal carcinoma with glandular (a), and papillary (b) growth pattern (20X)
Yolk Sac Tumor
Yolk sac tumor is the most common testicular neoplasm in children and occurs in all races: 80 % of pure yolk sac tumors occur in the first 2 years of life. Unlike the germ cell tumors of older patients, those in children have had a steady incidence over the years and are not associated with IGCNU. Pure yolk sac tumor in adults is uncommon, comprising approximately 1.5 % of testicular germ cell tumors; however, yolk sac tumor is found as a component of ~ 40 % of mixed germ cell tumors. The age of incidence in adults corresponds to that of patients with testicular mixed germ cell tumors. Serum alpha fetoprotein (AFP) levels are elevated in 90 % of cases.
On gross examination, the tumor is typically solid and soft, white-gray or light yellow with areas of cystic degeneration (Fig. 35.10). Large tumors may show necrosis and hemorrhage. Several microscopic growth patterns have been described, with the microcystic or reticular pattern being the most common one (Fig. 35.11a) [4, 6, 8] and consisting of sheets of prominently vacuolated tumor cells (lipoblast-like) or an anastomosing network of flattened neoplastic cells in a loose stroma. Other patterns include macrocystic, papillary (Fig. 35.11b), glandular, endodermal sinus, solid, myxomatous (Fig. 35.11c), polyvesicular vitelline, hepatoid, enteric, and parietal. Schiller-Duval bodies and eosinophilic hyaline globules are highly characteristic of yolk sac tumor. The former consist of solitary papillae with a central vascular core enveloped by endodermal epithelium. Hyaline globules are non-membrane-bound cytoplasmic and extracellular globules of uncertain composition. Another characteristic finding is bandlike deposits of extracellular basement membrane between tumor cells, so-called parietal differentiation of the tumor.
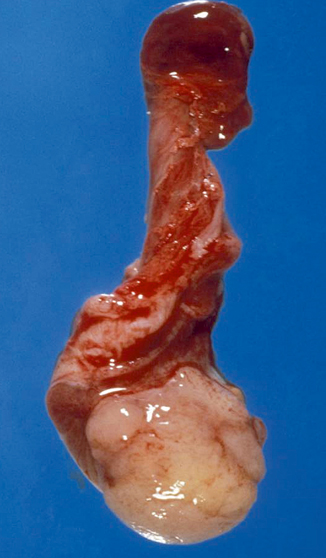
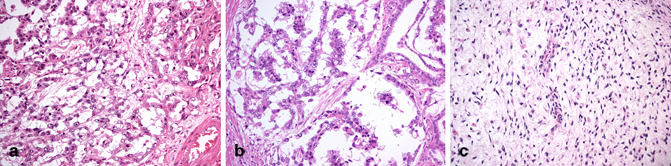

Fig. 35.10
Yolk sac macroscopically characterized by a solid and soft, light gray-yellow tumor

Fig. 35.11
Yolk sac tumor with microcystic or reticular pattern (a), papillary pattern (b), and myxomatous pattern (c) (20X)
The tumor cells stain for low molecular weight cytokeratin (but not cytokeratin 7), PLAP, glypican 3 and focally for AFP, but are negative for OCT3/4. The solid variant of yolk sac tumor, which may be confused with seminoma, is discussed in Chap. 37.
Choriocarcinoma
Choriocarcinoma is very rare (< 1 %) as a pure testicular germ cell tumor; much more often it is admixed with other germ cell tumor elements. It occurs in young patients (mean age 25–30 years) who commonly present with symptoms related to metastatic disease. The patients typically have elevated levels of serum HCG. Grossly, it commonly forms a hemorrhagic and necrotic nodule (Fig. 35.12).
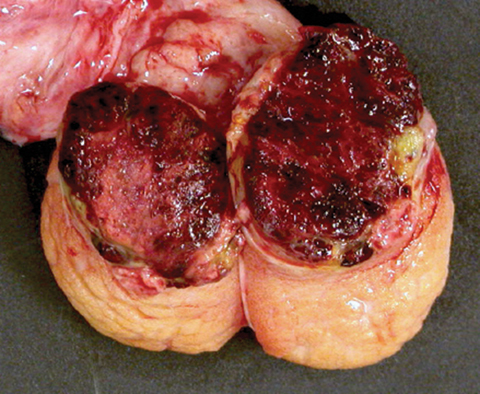

Fig. 35.12
Testis largely replaced by a hemorrhagic nodule of choriocarcinoma
Histologically choriocarcinoma is composed of an admixture of syncytiotrophoblastic, cytotrophoblastic, and intermediate trophoblastic cells (Fig. 35.13a, b). The cytotrophoblastic cells are mononucleated with pale to clear cytoplasm, marked nuclear atypia, and one or two prominent nucleoli. The syncytiotrophoblastic cells are multinucleated with abundant eosinophilic to basophilic cytoplasm; they typically have several, large, irregularly shaped, hyperchromatic nuclei that frequently have a “smudged” appearance (Fig. 35.13a). Intermediate trophoblastic cells have eosinophilic to clear cytoplasm and single nuclei. The background is extensively hemorrhagic and necrotic [4, 6] (Fig. 35.13b).
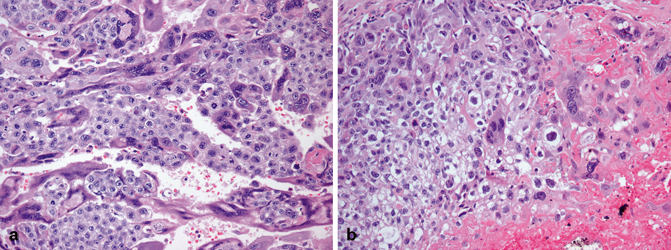

Fig. 35.13
Choriocarcinoma composed of an admixture of syncytiotrophoblastic, cytotrophoblastic (a), and intermediate trophoblastic cells in a hemorrhagic background (b)
The syncytiotrophoblastic cells express HCG, but the cytotrophoblastic ones show weak to no staining. All cell types express cytokeratin, and about half of the cases express EMA and PLAP. Inhibin is also typically positive .
Teratoma
Teratoma occurs in two age groups: in children the incidence ranges from 24 to 36 %; in adults pure teratoma accounts for 2.7–7 % of testicular germ cell tumors , but a teratomatous component is detected in approximately half of germ cell tumors. Teratoma is the second most common germ cell tumor in young children (first and second year of life), in whom it is invariably benign. On the other hand, almost all tumors occurring at postpubertal ages (young adults) have a malignant potential, even when histologically mature [8]. Most lesions are nodular and firm and have a heterogeneous cut surface with solid and cystic areas (Fig. 35.14). Cartilage, bone, and pigmented areas may be recognizable.
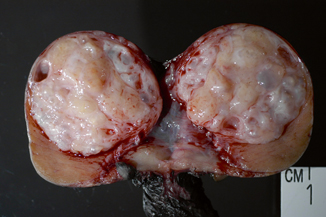

Fig. 35.14
Teratoma with heterogeneous cut surface with solid and cystic areas
Mature teratoma is composed of well-differentiated somatic tissues resembling those seen at other body sites, including squamous, enteric, and respiratory epithelium, cartilage, and muscular tissue. Immature teratoma, in addition to mature elements, contains incompletely differentiated tissues resembling those of embryonic development including immature neuroectoderm, Wilms tumor-like blastema and stroma and rhabdomyoblastic cells. The teratomas in the postpubertal group commonly show cytologic atypia and disorganized arrangements of elements, whereas those in the prepubertal patients are cytologically bland and frequently organoid. This disparity in morphology reflects their derivation from either IGCNU (postpubertal group) or a non-transformed, benign germ cell (prepubertal group). The presence of immature elements does not alter the behavior of the postpubertal tumors, and it is therefore not considered necessary to make a distinction between mature and immature teratoma. Teratoma with a secondary malignant component is characterized by overgrowth of a malignancy resembling those seen at somatic sites, either a sarcoma, or a carcinoma, or both. A secondary sarcoma (such as primitive neuroectodermal tumor or PNET) arising in a teratoma should be considered when the sarcoma forms a nodule equal to or greater than a 4X objective microscopic field (Fig. 35.15).
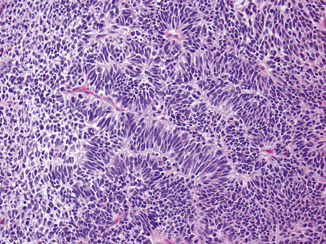

Fig. 35.15
Primitive neuroectodermal tumor (PNET) arising in a teratoma
Epidermoid cyst , dermoid cyst (mature cystic teratoma), and non-dermoid benign teratoma are discussed in Chap. 37.
Sex Cord/Gonadal Stromal Tumors
Testicular stromal tumors are rare and account for only 2–4 % of adult testicular tumors.
Leydig Cell Tumor
Leydig cell tumors are the most common type of sex cord/gonadal stromal tumor, accounting for 1–3 % of adult testicular neoplasms [9] and 3 % of tumors in infants and children. Approximately 3 % of Leydig cell tumors are bilateral [10]. They exhibit a peak incidence in preadolescent children (3–9 years old) as well as in the older (third to sixth decade) age groups.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


