Childhood Melanoma
Jeremy C. Wallentine, MD
Brian Hall, MD
Key Facts
Clinical Issues
Knowledge of clinical dimensions is of maximum importance
Ensure viewing of entire lesion before making a benign diagnosis
Can be difficult, especially in cases of giant congenital melanocytic nevus (CMN)
Microscopic Pathology
Asymmetry of lesion
One of most powerful criteria for diagnosing melanoma
Lack of circumscription
Pagetoid spread of single melanocytes above basal layer, especially at periphery of lesion
Lack of maturation
Lack of dispersion
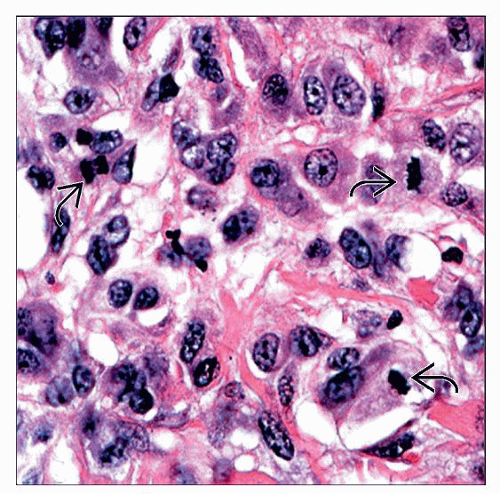
Deep dermal mitoses
Pigment deep in lesion
Solitary epidermal melanocytes predominating over nests
Reporting Considerations
Breslow thickness, not histologic subtype, is most important prognostic parameter
Presence or absence of ulceration changes stage
Malpractice Considerations
Expert consultation recommended before diagnosing melanoma in any pediatric patient
Due to wide range of histologic features and subtypes of melanomas
Diagnosis of melanoma should be considered when encountering any unusual cutaneous malignancy
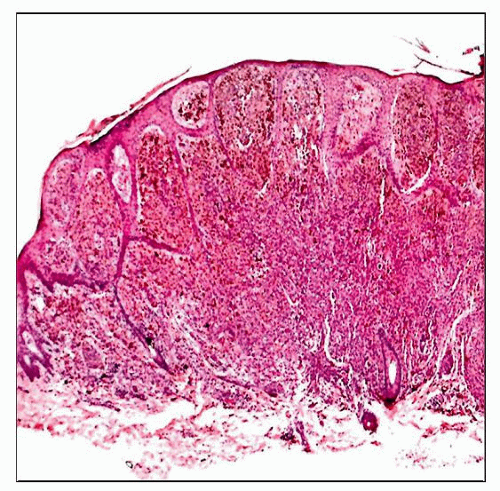 A nevoid melanoma arising in a 4-year-old child is shown. Although the cells appear to show some maturation at low magnification, there were deep atypical cells and mitoses present on high power. |
TERMINOLOGY
Abbreviations
Malignant melanoma (MM)
Synonyms
Melanoma of childhood
Definitions
Malignant cutaneous melanocytic neoplasm arising in children
ETIOLOGY/PATHOGENESIS
Classification of Pediatric Melanoma
Classified according to mode of occurrence and histology
Transformation from giant congenital melanocytic nevus (CMN)
In association with congenital predisposing conditions
Development from preexisting nevus
Transplacental melanoma
CLINICAL ISSUES
Epidemiology
Incidence
Accounts for 1-3% of all childhood malignancies
Accounts for < 0.5% of all melanomas
7x more frequent in 2nd decade than 1st decade of life
On the rise in children and teenagers
Age
Prepubescent melanoma
Congenital and infantile melanomas are rare
Develops transplacentally, de novo, or within a CMN, especially giant congenital nevi
Postpubescent melanoma
> 14 years of age
Clinical features and prognosis tend to resemble adult counterparts
Gender
Slight female predominance
Site
Can occur anywhere on the skin
Rarely mucous membranes and meninges
Presentation
50% arise in association with preexisting lesion
30% arise within a giant CMN (> 20 cm)
50-70% before puberty
Tend to arise within dermis
Worse prognosis
20% in association with other cutaneous nevi
Small to medium-sized CMNs
Acquired melanocytic nevi
More likely to occur after puberty
50% arise de novo
May arise within neurocutaneous melanosis
Rare but carries a high risk of malignant transformation in children
Median age is 3 years old
Up to 2/3 of patients may develop primary intracranial melanomas
Signs and symptoms may include
Rapid increase in size of lesion, hemorrhage, ulceration, change in color, loss of previously regular borders, pruritus, lymphadenopathy
Important clinical signs (“ABCD” criteria)
Asymmetry
Border irregularity
Color/pigmentation irregularities
Diameter of 6 mm or greater
Risk factors
Fair skin
Giant CMN (bathing trunk nevus)
Risk correlates with size, depth, and number of nevomelanocytes
Occurs in 1 in 20,000 newborns
≥ 20 cm in largest diameter
Up to 5-7% risk of malignant transformation
Dysplastic nevus syndrome
Numerous acquired melanocytic nevi
Independent risk factor
Sporadic atypical nevi
Independent risk factor
Xeroderma pigmentosum
Albinism
Immunosuppression
Family history of melanoma (familial melanoma)
Occur at younger age
Often multifocal
Germline mutations of CDKN2A tumor suppressor gene
Treatment
Options, risks, complications
Surgical resection with standard margins
Treatment of choice for primary disease
Potentially curative
May also include sentinel lymph node biopsy or regional lymphadenectomy
Both the NCCN and AAD publish online guidelines for surgical margins
Chemotherapy of minimal benefit
Experimental immunotherapy of unproven benefit
Treatment protocols based on adult population
Prognosis
Most important prognostic factors
Depth of invasion
Clark level and Breslow thickness
Most accurately measured by Breslow thickness
Clark level is of questionable significance
Stage at diagnosis
Stage IV 5-year survival rate (34%)
Stage I-II 5-year survival rate (90%)
Other poor prognostic indicators
Previous nonmelanocytic malignancies, nodular type, fusiform cytology, vertical growth phase
High dermal mitotic activity, ulceration, vascular invasion, age > 10 years, and presence of metastases at diagnosis
Presence of ulceration upstages tumor (e.g., T2a to T2b)
Overall 5-year survival ~ 79%
Survival characteristics similar to adult population
MACROSCOPIC FEATURES
Size
Usually > 7 mm
MICROSCOPIC PATHOLOGY
Histologic Features
May or may not have ulceration
Asymmetry
One of the most powerful histologic criteria
Nests showing
Variability in size and shape
Haphazard interval and array
Haphazard arrangement of solitary epidermal melanocytes
Poor circumscription
Lesion does not start or end in nests
Difficult to discern where lesion begins and ends
Single melanocytes predominate at edge of lesion
Solitary epidermal melanocytes predominate over nests
Pagetoid spread of melanocytes
Ascent of single melanocytes above dermoepidermal (DE) junction
Can also be present in Spitz nevi (sometimes full nests) and acral nevi
Should not be at the periphery of Spitz or acral nevi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree