Chemotherapy of Protozoal Infections: Amebiasis, Giardiasis, Trichomoniasis, Trypanosomiasis, Leishmaniasis, and Other Protozoal Infections
Humans host a wide variety of protozoal parasites that can be transmitted by insect vectors, directly from other mammalian reservoirs or from one person to another. The immune system plays a crucial role in protecting against the pathological consequences of protozoal infections. Thus, opportunistic infections with protozoa are prominent in infants, individuals with cancer, transplant recipients, those receiving immunosuppressive drugs or extensive antibiotic therapy, and persons with advanced human immunodeficiency virus (HIV) infection. Because effective vaccines are unavailable, chemotherapy has been the only practical way to both treat infected individuals and reduce transmission. Many effective antiprotozoal drugs are toxic at therapeutic doses, a problem exacerbated by increasing drug resistance.
INTRODUCTION TO PROTOZOAL INFECTIONS OF HUMANS
AMEBIASIS. Amebiasis affects ~10% of the world’s population, commonly individuals living in poverty, crowded conditions, and areas with poor sanitation. Three morphologically identical but genetically distinct species of Entamoeba (i.e., E. histolytica, E. dispar, and E. moshkovskii) have been isolated from infected persons, with E. histolytica responsible for ~10% of human infections. Only E. histolytica causes disease and requires treatment.
Humans are the only known hosts for these protozoa, which are transmitted almost exclusively by the fecal-oral route. Ingested E. histolytica cysts from contaminated food or water survive acid gastric contents and transform into trophozoites that reside in the large intestine. The outcome of E. histolytica infection is variable. Many individuals remain asymptomatic but excrete the infectious cyst form, making them a source for further infections. In other individuals, E. histolytica trophozoites invade into the colonic mucosa with resulting colitis and bloody diarrhea (amebic dysentery). In a smaller proportion of patients, E. histolytica trophozoites invade through the colonic mucosa, reach the portal circulation, and travel to the liver, where they establish an amebic liver abscess.
The cornerstone of therapy for amebiasis is metronidazole or its analogs tinidazole and ornidazole. Because metronidazole is so well absorbed in the gut, levels may not be therapeutic in the colonic lumen, and the drug is less effective against cysts. Hence patients with amebiasis (amebic colitis or amebic liver abscess) also should receive a luminal agent to eradicate any E. histolytica trophozoites residing within the gut lumen. Luminal agents are also used to treat asymptomatic individuals found to be infected with E. histolytica. The nonabsorbed aminoglycoside paromomycin and the 8-hydroxyquinoline compound iodoquinol are effective luminal agents. Diloxanide furoate, previously considered the luminal agent of choice for amebiasis, is no longer available in the U.S. Nitazoxanide (ALINIA), approved in the U.S. for treatment of cryptosporidiosis and giardiasis, is also active against E. histolytica.
GIARDIASIS. Giardiasis, caused by the flagellated protozoan Giardia intestinalis, is prevalent worldwide and is the most commonly reported intestinal protozoal infection in the U.S. Infection results from ingestion of the cyst form of the parasite, which is found in fecally contaminated water or food.
Infection with Giardia results in 1 of 3 syndromes: an asymptomatic carrier state, acute self-limited diarrhea, or chronic diarrhea. Chemotherapy with a 5-day course of metronidazole usually is successful, although therapy may have to be repeated or prolonged in some instances. A single dose of tinidazole (TINDAMAX, others) probably is superior to metronidazole for the treatment of giardiasis. Paromomycin (HUMATIN, others) has been used to treat pregnant women to avoid any possible mutagenic effects of the other drugs. Nitazoxanide (ALINIA), N-(nitrothiazolyl) salicylamide, and tinidazole are approved for the treatment of giardiasis in immune-competent children <12 years of age. Furazolidone has been discontinued in the U.S.
TRICHOMONIASIS. Trichomoniasis is caused by the flagellated protozoan Trichomonas vaginalis. This organism inhabits the genitourinary tract of the human host, where it causes vaginitis in women and, uncommonly, urethritis in men. Trichomoniasis is a sexually transmitted disease and has been associated with an increased risk of acquiring HIV infection. Only trophozoite forms of T. vaginalis have been identified in infected secretions.
Metronidazole remains the drug of choice for the treatment of trichomoniasis. Tinidazole, another nitroimidazole, appears to be better tolerated than metronidazole and has been used successfully at higher doses to treat metronidazole-resistant T. vaginalis.
TOXOPLASMOSIS. Toxoplasmosis is a zoonotic infection caused by Toxoplasma gondii. Although cats and other feline species are the natural hosts, tissue cysts (bradyzoites) have been recovered from all mammalian species examined. Common routes of infection in humans are:
• Ingestion of undercooked meat containing tissue cysts
• Ingestion of contaminated vegetable matter containing infective oocysts
• Direct oral contact with feces of cats shedding oocysts
• Transplacental fetal infection with tachyzoites from acutely infected mothers
The acute illness is usually self-limiting, and treatment rarely is required. Individuals who are immunocompromised, however, are at risk of developing toxoplasmic encephalitis from reactivation of tissue cysts deposited in the brain. The primary treatment for toxoplasmic encephalitis consists of the antifolates pyrimethamine (DARAPRIM) and sulfadiazine along with folinic acid (leucovorin). Therapy must be discontinued in ~40% of cases because of toxicity owing primarily to the sulfa compound; clindamycin can be substituted for sulfadiazine without loss of efficacy. Alternative regimens combining azithromycin, clarithromycin, atovaquone, or dapsone with either trimethoprim-sulfamethoxazole or pyrimethamine and folinic acid are less toxic but also less effective. Spiramycin, which concentrates in placental tissue, is used for the treatment of acute acquired toxoplasmosis in pregnancy to prevent transmission to the fetus. If fetal infection is detected, the combination of pyrimethamine, sulfadiazine, and folinic acid is administered to the mother (only after the first 12-14 weeks of pregnancy) and to the newborn in the postnatal period. Spiramycin is not available in the U.S.
CRYPTOSPORIDIOSIS. Cryptosporidia are coccidian protozoan parasites that can cause diarrhea. Cryptosporidium parvum and the newly named C. hominis appear to account for almost all infections in humans. Infectious oocysts in feces may be spread either by direct human-to-human contact or by contaminated water supplies.
After ingestion, the mature oocyte is digested, releasing sporozoites that invade host epithelial cells. In most individuals, infection is self-limited. However, in AIDS patients and other immunocompromised individuals, the severity of diarrhea may require hospitalization. The most effective therapy for cryptosporidiosis in AIDS patients is restoration of their immune function through highly active antiretroviral therapy (HAART) (see Chapter 59). Nitazoxanide has shown activity in treating cryptosporidiosis in immunocompetent children and is possibly effective in immunocompetent adults. Its efficacy in children and adults with HIV infection and AIDS is not clearly established.
TRYPANOSOMIASIS. African trypanosomiasis, or “sleeping sickness,” is caused by subspecies of the hemoflagellate Trypanosoma brucei that are transmitted by bloodsucking tsetse flies of the genus Glossina. Largely restricted to sub-Saharan Africa, the infection causes serious human illness and also threatens livestock (nagana), leading to protein malnutrition. In humans, the infection is fatal unless treated. An estimated 500,000 Africans carry the infection, and >50 million people are at risk for the disease.
The parasite is entirely extracellular, and early human infection is characterized by the finding of replicating parasites in the bloodstream or lymph without CNS involvement (stage 1); stage 2 disease is characterized by CNS involvement. Symptoms of early-stage disease include febrile illness, lymphadenopathy, splenomegaly, and occasional myocarditis that result from systemic dissemination of the parasites. There are 2 types of African trypanosomiasis: the East African (Rhodesian; T. brucei rhodesiense) variety produces a progressive and rapidly fatal form of disease marked by early involvement of the CNS and frequent terminal cardiac failure; the West African type (Gambian; T. brucei gambiense) causes illness characterized by later involvement of the CNS and a more long-term course that progresses to the classical symptoms of sleeping sickness over months to years. Neurological symptoms include confusion, sensory deficits, psychiatric signs, disruption of the sleep cycle, and eventual progression into coma and death.
Standard therapy for early-stage disease is pentamidine for T. brucei gambiense and suramin for T. brucei rhodesiense. Both compounds must be given parenterally over long periods and are not effective against late-stage disease. The CNS phase has traditionally been treated with melarsoprol (available from the CDC), a highly toxic agent that causes a fatal reactive encephalopathy in 2-10% of treated patients. Moreover, lack of response to this agent is leading to increasing numbers of treatment failures. Eflornithine, an inhibitor of ornithine decarboxylase, a key enzyme in polyamine metabolism, offers the only alternative for the treatment of late-stage disease. It has efficacy against both early and late stages of human T. brucei gambiense infection; however, it is ineffective as monotherapy for infections of T. brucei rhodesiense. Notably, eflornithine has significantly fewer side effects than melarsoprol and is more effective than melarsoprol for treatment of late-stage Gambian trypanosomiasis, suggesting that eflornithine is the best available first-line treatment for this form of the disease. Nifurtimox-eflornithine combination therapy (NECT) allows a shorter exposure to eflornithine with good efficacy and a reduction in adverse events.
American trypanosomiasis, or Chagas disease, a zoonotic infection caused by Trypanosoma cruzi, affects ~15 million people from Mexico to Argentina and Chile. The chronic form of the disease in adults is a major cause of cardiomyopathy, megaesophagus, megacolon, and death. Blood-sucking triatomid bugs infesting poor rural dwellings most commonly transmit this infection to young children; transplacental transmission also may occur in endemic areas. Two nitroheterocyclic drugs, nifurtimox (available from the CDC) and benznidazole are used to treat this infection. Both agents suppress parasitemia and can cure the acute phase of Chagas disease in 60-80% of cases; both drugs are toxic and must be taken for long periods.
LEISHMANIASIS. Leishmaniasis is a complex vector-borne zoonosis caused by ~20 different species of intramacrophage protozoa of the genus Leishmania. Small mammals and canines generally serve as reservoirs for these pathogens, which can be transmitted to humans by the bites of female phlebotomine sandflies.
In increasing order of systemic involvement and potential clinical severity, major syndromes of human leishmaniasis have been classified into cutaneous, mucocutaneous, diffuse cutaneous, and visceral (kala azar) forms. Cutaneous forms of leishmaniasis generally are self-limiting, with cures occurring in 3-18 months after infection. However, this form of the disease can leave disfiguring scars. The mucocutaneous, diffuse cutaneous, and visceral forms of the disease do not resolve without therapy. Visceral leishmaniasis caused by L. donovani is fatal unless treated. The classic therapy for all species of Leishmania is pentavalent antimony (sodium antimony gluconate; sodium stibogluconate; PENTOSTAM); resistance to this compound is widespread in India, although it remains useful in other parts of the world. As an alternative, liposomal amphotericin B is a highly effective agent for visceral leishmaniasis, and it is currently the drug of choice for antimony-resistant disease. Treatment of leishmania has undergone major changes owing to the success of the first orally active agent, miltefosine. The drug also appears to have promise for the treatment of the cutaneous disease and for the treatment of dogs, an important animal reservoir of the disease. Paromomycin has been used with success as a parenteral agent for visceral disease, and topical formulations of paromomycin have efficacy against cutaneous disease.
OTHER PROTOZOAL INFECTIONS. Just a few of the many less common protozoal infections of humans are highlighted here.
Babesiosis, caused by either Babesia microti or B. divergens, is a tick-borne zoonosis that superficially resembles malaria in that the parasites invade erythrocytes, producing a febrile illness, hemolysis, and hemoglobinuria. This infection usually is mild and self-limiting but can be severe or even fatal in asplenic or severely immunocompromised individuals. Currently recommended therapy is with a combination of clindamycin and quinine for severe disease, and the combination of azithromycin and atovaquone for mild or moderate infections.
Balantidiasis, caused by the ciliated protozoan Balantidium coli, is an infection of the large intestine that may be confused with amebiasis. Unlike amebiasis, this infection usually responds to tetracycline therapy.
Isospora belli, a coccidian parasite, causes diarrhea in AIDS patients and responds to treatment with trimethoprim-sulfamethoxazole. Cyclospora cayetanensis causes self-limited diarrhea in normal hosts and can cause prolonged diarrhea in individuals with AIDS.
Microsporidia are spore-forming unicellular eukaryotic fungal parasites that can cause a number of disease syndromes, including diarrhea in immunocompromised individuals. Infections with microsporidia have been treated successfully with albendazole, an inhibitor of β-tubulin polymerization (see Chapter 51). Immunocompromised individuals with intestinal microsporidiosis due to E. bieneusi (which does not respond as well to albendazole) have been treated successfully with the antimicrobial fumagillin.
ANTIPROTOZOAL DRUGS
For ease of reference, the myriad agents used to treat nonmalarial protozoal diseases are presented alphabetically.
AMPHOTERICIN B
The pharmacology, formulation, and toxicology of amphotericin B are presented in Chapter 57.
Antiprotozoal Effects. Amphotericin B (AMBISOME) is a highly effective antileishmanial agent that cures >90% of the cases of visceral leishmaniasis and is the drug of choice for antimonial-resistant cases. It is a second-line drug for cutaneous or mucosal leishmaniasis, where it has been shown effective for the treatment of immunocompromised patients. The lipid preparations of the drug have reduced toxicity, but the cost of the drug and the difficulty of administration remain a problem in endemic regions.
Mechanism of Action. The basis of amphotericin B action against leishmania is similar to that for the drug’s antifungal activities (see Chapter 57). Amphotericin complexes with ergosterol precursors in the cell membrane, forming pores that allow ions to enter the cell. Leishmania has similar sterol composition to fungal pathogens, and the drug binds to these sterols preferentially over the host cholesterol. No significant resistance to the drug has been encountered after nearly 30 years of use as an antifungal agent.
Therapeutic Uses. Typical regimens of 10-20 mg/kg total dose given in divided doses over 10-20 days by intravenous infusion have yielded >95% cure rates. In the U.S., the FDA recommends 3 mg/kg intravenously on days 1-5, 14, and 21 for a total dose of 21 mg/kg. Recent data suggest that a single dose of 5 mg/kg followed by 7-14 days treatment with oral miltefosine was effective at curing visceral leishmaniasis, and this dosing scheme warrants additional study.
CHLOROQUINE
The pharmacology and toxicology of chloroquine are presented in Chapter 49 (antimalarials). Chloroquine does have an FDA-approved use for extra-intestinal amebiasis at a dose of 1 g (600 mg base) daily for 2 days, followed by 500 mg daily for at least 2-3 weeks. Treatment is usually combined with an effective intestinal amebicide.
DILOXANIDE FUROATE
Diloxanide furoate (FURAMIDE, others) is a derivative of dichloroacetamide. Diloxanide furoate is a very effective luminal agent for the treatment of E. histolytica infection but is no longer available in the U.S.
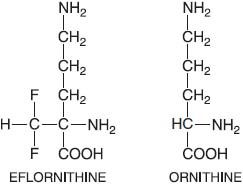
EFLORNITHINE
Eflornithine (α-D,L difluoromethylornithine, DFMO, ORNIDYL) is an irreversible catalytic (suicide) inhibitor of ornithine decarboxylase, the enzyme that catalyzes the first and rate-limiting step in the biosynthesis of polyamines (putrescine, spermidine, and spermine) that are required for cell division and for normal cell differentiation. In trypanosomes, spermidine is required for the synthesis of trypanothione, a conjugate of spermidine and glutathione that replaces many of the functions of glutathione in the parasite.
Eflornithine currently is used to treat West African (Gambian) trypanosomiasis caused by T. brucei gambiense; the drug is largely ineffective for East African trypanosomiasis. Eflornithine’s difficult treatment regimen limits its use. Eflornithine is no longer available for systemic use in the U.S. but is available for treatment of Gambian trypanosomiasis by special request from the CDC. Eflornithine is safer and more efficacious than melarsoprol for late-stage gambiense sleeping sickness, and is the recommended first-line treatment for this disease when adequate care can be provided for its administration.
Antitrypanosomal Effects. Eflornithine is a cytostatic agent that has multiple biochemical effects on trypanosomes, all of which are a consequence of polyamine depletion. The parasite and human enzymes are equally susceptible to inhibition by eflornithine; however, the mammalian enzyme is turned over rapidly, whereas the parasite enzyme is stable, and this difference likely plays a role in the selective toxicity. T. brucei rhodesiense cells are less sensitive to eflornithine inhibition than T. brucei gambiense cells; studies in vitro suggest that the effective doses are increased by 10-20 times in the refractory cells. The molecular basis for the higher dose requirement in T. brucei rhodesiense is not understood.
ADME. Eflornithine is given by intravenous infusion. The drug does not bind to plasma proteins but is well distributed and penetrates into the CSF, where estimated concentrations of at least 50 μM must be reached to clear well distributed and penetrates into the CSF. Renal clearance after intravenous administration is rapid (2 mL/min/kg), with >80% of the drug cleared by the kidney largely in unchanged form. Some studies indicate that suramin enhances eflornithine uptake into the CNS and could lower the dose requirements for eflornithine.
Therapeutic Uses. Eflornithine is used for the treatment of late-stage West African trypanosomiasis caused by T. brucei gambiense
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


