Chemical stability in dosage forms
Andrew R. Barnes
Chapter contents
Chemical degradation reactions
Dimerization and polymerization
Stability of proteins and peptides
Physical stability of proteins
Chemical aspects of protein stability
Chemical modification of protein stability
Key points
Introduction
Chemical degradation of the drug is often the critical factor that limits the shelf-life of a pharmaceutical product. The degradation of other ingredients in the formulation, such as antimicrobial preservatives or antioxidants, may also be a critical factor.
The nature of the degradation products that form in the dosage form may be the factor that limits the shelf-life of a product. This may be because the degradation products are toxic; for instance, the antifungal drug flucytosine degrades to fluorouracil, which is cytotoxic. The degradation products may alternatively give the product an unacceptable appearance. For instance, the oxidation products of epinephrine (adrenaline) are highly coloured.
In general, drug molecules tend not to undergo spontaneous chemical degradation; the cause is usually some other reactive molecule within the dosage form. Often, this is due to the presence of water or molecular oxygen, but the drug may also react with other formulation constituents or react with other molecules of the same drug. Protection of the formulation from chemical degradation is one of the primary aims of dosage form design.
This chapter discusses the common types of chemical degradation that affect ‘small molecule’ drugs and then looks at the specific stability issues affecting proteins and peptides.
Chemical degradation reactions
Hydrolysis
Hydrolysis is the breaking of a molecular bond by reaction with water. Water is common in pharmaceutical products, either as an ingredient or as a contaminant, and hydrolysis reactions are the most common cause of chemical degradation. Most hydrolysis reactions involve derivatives of carboxylic acids, such as esters and amides, which are frequently found in drug molecules.
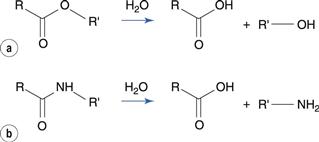
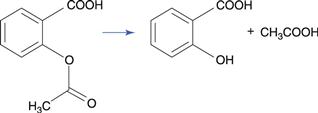
The ester group hydrolyses to produce a carboxylic acid and an alcohol (Fig. 48.1a). The carbon of the ester carboxyl group is relatively electron deficient, due to bond polarization caused by the adjacent oxygen atoms. Nucleophilic attack by water is therefore promoted at this carbon atom. For instance, the degradation of aspirin (acetylsalicylic acid) results in the formation of salicylic acid and acetic acid (Fig. 48.2). Aspirin is too unstable to allow the formulation of an aqueous aspirin product with a suitable shelf-life.
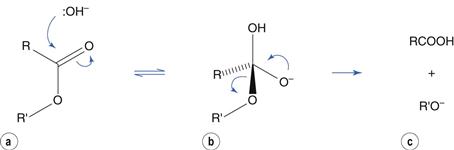
Hydrolysis reactions of esters, amides and related molecules are catalyzed by acid and by base. To use ester hydrolysis as an example, catalysis by base involves nucleophilic attack by hydroxyl ion at the electron-deficient carbon of the carbonyl group to produce a tetrahedral intermediate (Fig. 48.3a,b). This is followed by ejection of the alcohol (Fig. 48.3c). In this scheme, ionization of the carboxylic acid in alkaline solution is ignored for clarity.
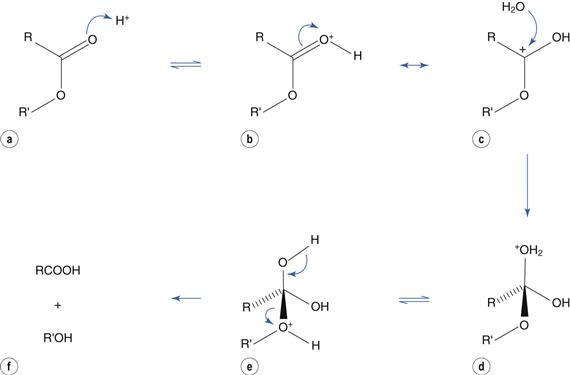
Catalysis by acid involves protonation of the carbonyl group (Fig. 48.4a) to produce resonance structures (Fig. 48.4b, c). The positively-charged carbon atom promotes nucleophilic attack by water (Fig. 48.4c) to produce a tetrahedral intermediate (Fig. 48.4d). Transfer of H+ within the molecule (Fig. 48.4e) results in loss of the alcohol portion (Fig. 48.4f).
Amides degrade to a carboxylic acid and an amine (Fig. 48.1b). Amides tend to be more stable to hydrolysis than the corresponding esters because the nitrogen atom is less electronegative than the oxygen atom in the corresponding ester. Examples of amide-containing drugs include lidocaine and paracetamol (acetaminophen). The antimicrobial drug chloramphenicol is an amide-containing drug that is relatively susceptible to hydrolysis compared to many amides (Fig. 48.5). This is due to a high degree of polarization of the amide bond by the highly electronegative chlorine substituents that are adjacent. Eye drop preparations of chloramphenicol therefore require storage in a refrigerator.
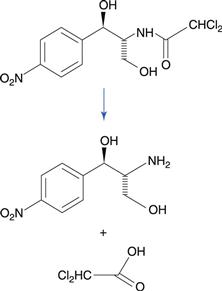
Fig. 48.5 Hydrolysis of chloramphenicol to 2-amino-1-(4-nitrophenyl)propane-1.3-diol and dichloroacetic acid.
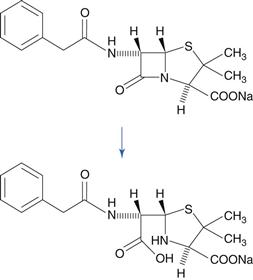
The lactam group, which is a cyclic amide, is important because it is present in penicillin and cephalosporin antibiotics, and this group is very susceptible to hydrolysis. This reactivity of the molecule is due to bond strain in the four-membered β-lactam ring. A variety of hydrolysis products is formed. For benzylpenicillin, a major hydrolysis product is benzylpenicilloic acid (Fig. 48.6). Penicillins have a side chain that possesses an amide group, but this is less susceptible to hydrolysis than the β-lactam ring. Benzylpenicillin cannot be administered by the oral route because it is hydrolysed rapidly by the acidic conditions in the stomach. Orally-active penicillins such as amoxicillin are relatively less susceptible to hydrolysis.
Oxidation
Oxidation reactions involve an increase in the number of carbon-to-oxygen bonds in a molecule or a reduction in the number of carbon-to-hydrogen bonds. These reactions are a common cause of chemical instability of drugs. They are also responsible for the deterioration of vegetable oils, which may be used in pharmaceutical products as a solvent or as an emollient in emulsions and creams. Oxidation reactions tend to be complex, giving a variety of degradation products. Table 48.1 summarizes common examples.
Oxidation that takes place at ambient temperature and involves molecular oxygen is known as autoxidation. Most such reactions involve free radicals, which are chemical species that possess an unpaired electron. Free radical oxidations are often complex but involve three main phases. The following scheme is a representative summary of the oxidation of many drugs and of vegetable oils.
The initiation phase results in the formation of a low concentration of free radicals. For a drug, RH, the generation of free radicals can be represented as:
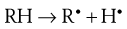
Initiation is promoted by light and the presence of heavy metals, which are inevitably found as trace contaminants of pharmaceutical products.
During the propagation phase, the concentration of free radicals increases greatly:
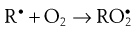
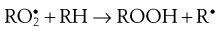
The presence of oxygen results in the formation of hydroperoxides (ROOH), which react further to produce stable oxidation products. In this phase, degradation accelerates, with potentially disastrous results for the product.
In the termination phase
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree