Central Nervous System Infections
KEY CONCEPTS
![]() The four most likely pathogens of bacterial meningitis in the United States are Streptococcus pneumoniae, group B Streptococcus, Neisseria meningitidis, and Haemophilus influenzae type b, although routine vaccination is having a dramatic effect on the incidence of these pathogens causing infection.
The four most likely pathogens of bacterial meningitis in the United States are Streptococcus pneumoniae, group B Streptococcus, Neisseria meningitidis, and Haemophilus influenzae type b, although routine vaccination is having a dramatic effect on the incidence of these pathogens causing infection.
![]() In cases of meningitis, initial findings can include (a) presenting signs and symptoms: fever, headache, nuchal rigidity (the classic triad), Brudzinski’s or Kernig’s sign, and altered mental status; and (b) abnormal cerebrospinal fluid (CSF) chemistries: elevated white blood cell (WBC) count (>1,000 cells/mm3 [<1 × 109/L]), elevated protein (>50 mg/dL [>500 mg/L]), and decreased glucose levels (<45 mg/dL [<2.5 mmol/L).
In cases of meningitis, initial findings can include (a) presenting signs and symptoms: fever, headache, nuchal rigidity (the classic triad), Brudzinski’s or Kernig’s sign, and altered mental status; and (b) abnormal cerebrospinal fluid (CSF) chemistries: elevated white blood cell (WBC) count (>1,000 cells/mm3 [<1 × 109/L]), elevated protein (>50 mg/dL [>500 mg/L]), and decreased glucose levels (<45 mg/dL [<2.5 mmol/L).
![]() Two main microbiologic tests that should be obtained include a Gram stain and culture of the CSF. Molecular testing such as polymerase chain reaction, latex coagglutination, and enzyme immunoassay (EIA) tests provide for the rapid identification of several causes of meningitis.
Two main microbiologic tests that should be obtained include a Gram stain and culture of the CSF. Molecular testing such as polymerase chain reaction, latex coagglutination, and enzyme immunoassay (EIA) tests provide for the rapid identification of several causes of meningitis.
![]() Three primary goals of treatment in meningitis include (a) eradication of infection, (b) amelioration of signs and symptoms, and (c) prevention of the development of neurologic sequelae, such as seizures, deafness, coma, and death.
Three primary goals of treatment in meningitis include (a) eradication of infection, (b) amelioration of signs and symptoms, and (c) prevention of the development of neurologic sequelae, such as seizures, deafness, coma, and death.
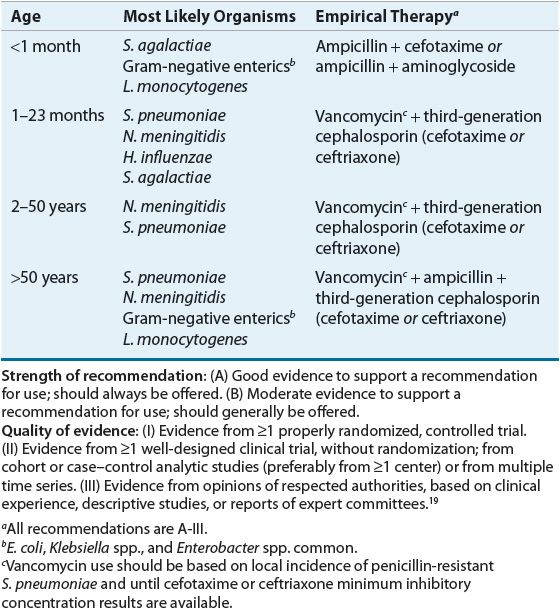
![]() When selecting antibiotics, the clinician must consider the antibiotic concentration at the site of infection, as well as the spectrum of antibacterial activity. Empirical choices should be based on age, predisposing conditions, and comorbidities. (a) Ceftriaxone or cefotaxime and vancomycin are reasonable initial choices for empirical coverage of community-acquired meningitis in adult patients. (b) Listeria monocytogenes is a common pathogen in infants and elderly; therefore, ampicillin with or without gentamicin should be added empirically to antimicrobial coverage.
When selecting antibiotics, the clinician must consider the antibiotic concentration at the site of infection, as well as the spectrum of antibacterial activity. Empirical choices should be based on age, predisposing conditions, and comorbidities. (a) Ceftriaxone or cefotaxime and vancomycin are reasonable initial choices for empirical coverage of community-acquired meningitis in adult patients. (b) Listeria monocytogenes is a common pathogen in infants and elderly; therefore, ampicillin with or without gentamicin should be added empirically to antimicrobial coverage.
![]() Empirical coverage with an appropriate antibiotic should be started as soon as possible when clinical suspicion of meningitis exists. If there is a delay in doing a lumbar puncture (even 30 to 60 minutes), or if the patient is to undergo neuroimaging, the first dose of an antibiotic should not be withheld. Changes in the CSF after initiation of antibiotics usually take 12 to 24 hours.
Empirical coverage with an appropriate antibiotic should be started as soon as possible when clinical suspicion of meningitis exists. If there is a delay in doing a lumbar puncture (even 30 to 60 minutes), or if the patient is to undergo neuroimaging, the first dose of an antibiotic should not be withheld. Changes in the CSF after initiation of antibiotics usually take 12 to 24 hours.
![]() Antibiotic dosages for the treatment of meningitis should be optimized to ensure adequate CNS penetration.
Antibiotic dosages for the treatment of meningitis should be optimized to ensure adequate CNS penetration.
![]() The duration of antibiotic treatment for meningitis has not been standardized; however, the duration generally is based on the causative organism and the individual case and may range from 7 to 21 days.
The duration of antibiotic treatment for meningitis has not been standardized; however, the duration generally is based on the causative organism and the individual case and may range from 7 to 21 days.
![]() Close contacts and relatives of the index case should be assessed for appropriate prophylaxis, particularly for N. meningitidis and H. influenzae meningitis.
Close contacts and relatives of the index case should be assessed for appropriate prophylaxis, particularly for N. meningitidis and H. influenzae meningitis.
![]() Steroid treatment includes dexamethasone 0.15 mg/kg per dose to be given four times daily for 4 days in infants and children older than 2 months of age with proven or strongly suspected bacterial meningitis. Steroids should be started prior to the first dose of antibiotics.
Steroid treatment includes dexamethasone 0.15 mg/kg per dose to be given four times daily for 4 days in infants and children older than 2 months of age with proven or strongly suspected bacterial meningitis. Steroids should be started prior to the first dose of antibiotics.
CNS infections are caused by a variety of pathogens, including bacteria, viruses, fungi, and parasites. Infections are the result of hematogenous spread from a primary infection site, seeding from a parameningeal focus, reactivation from a latent site, trauma, or congenital defects within the CNS. Newer diagnostic techniques have enabled more rapid and definitive diagnoses, thus diminishing the number of unknown “aseptic meningitis” diagnoses and improving targeted therapy. Bacteria resistant to multiple antibiotics present new challenges in the management of meningitis. This chapter presents the etiology, pathophysiology, therapy, and prophylaxis of these infections, concentrating predominantly on bacterial meningitis.
EPIDEMIOLOGY
Approximately 1.2 million cases of acute bacterial meningitis (ABM), excluding epidemics, occur every year around the globe, resulting in ~170,000 deaths.1,2 Mortality rates for patients with meningitis vary depending on the causative microorganism and age group. About 20% (range 12.3% to 35.3%) of survivors will experience one or more neurologic disabilities.3 Neurologic sequelae frequently associated with ABM include seizures, sensorineural hearing loss, and hydrocephalus. Risk for the development of neurologic sequelae depends on the infecting organism, with pneumococcal meningitis being associated with the highest risk.4 Despite the availability of antimicrobial therapy against the most common CNS pathogens, CNS infections continue to have significant morbidity and mortality.
Two findings that have the potential for great epidemiologic impact on bacterial meningitis are the following: (a) passive and active exposures to cigarette smoke are risk factors for bacterial meningitis, especially meningococcal disease,5 and (b) children with cochlear implants that include a positioner are at increased risk of bacterial meningitis, specifically pneumococcal meningitis. The incidence of meningitis due to Streptococcus pneumoniae in children with cochlear implants was more than 30 times the incidence in a similar cohort of the U.S. population without implants.6 Other risk factors for ABM include respiratory tract infection, otitis media mastoiditis, head trauma, alcoholism, high-dose steroids, splenectomy, sickle cell disease, immunoglobulin (Ig) deficiency, and immunosuppression.
ETIOLOGY
![]() CNS infections are caused by a variety of microorganisms. Historically, CNS infections were primarily community acquired; however, an increasing number of cases are now nosocomial.7 Haemophilus influenzae type b was the most commonly identified cause of bacterial meningitis until the introduction of the H. influenzae type b (Hib) conjugate vaccine in 1990, when S. pneumoniae became the most commonly identified cause in the United States (58%), followed by group B Streptococcus (GBS) (18.1%), Neisseria meningitidis (13.9%), H. influenzae (6.7%), and Listeria monocytogenes (3.4%).8
CNS infections are caused by a variety of microorganisms. Historically, CNS infections were primarily community acquired; however, an increasing number of cases are now nosocomial.7 Haemophilus influenzae type b was the most commonly identified cause of bacterial meningitis until the introduction of the H. influenzae type b (Hib) conjugate vaccine in 1990, when S. pneumoniae became the most commonly identified cause in the United States (58%), followed by group B Streptococcus (GBS) (18.1%), Neisseria meningitidis (13.9%), H. influenzae (6.7%), and Listeria monocytogenes (3.4%).8
Following the release of the heptavalent pneumococcal protein-conjugate vaccine (PCV7) in 2000, the rate of invasive pneumococcal disease steadily dropped from 24.3 cases per 100,000 people in 1999 to 17.3 per 100,000 in 2001 and 13.5 per 100,000 in 2007. The largest impact was in children younger than 2 years of age where a nearly 70% decline in infection rate was reported as a result of implementation in the routine childhood vaccination schedule. Interestingly, the effect carried into the adult population as well with significant reduction in invasive pneumococcal disease across all age groups.9,10
As a result of the decline of ABM rates in children, the median age of patients increased from 30.3 years in 1998 to 41.3 years in 2007 in the United States.8 Both the Hib and pneumococcal vaccines are of limited availability in developing countries where cost is often prohibitive. Thus, rate of invasive disease and case fatalities among children continue to be much higher than in Western countries.
ANATOMY AND PHYSIOLOGY OF THE CENTRAL NERVOUS SYSTEM
Meninges
The skull and vertebrae protect the CNS from blunt or penetrating trauma (Fig. 84–1). The brain is suspended in these structures by cerebrospinal fluid (CSF) and is surrounded by the meninges. The meninges are made up of three separate membranes: dura mater, arachnoid, and pia mater.11 Dura mater, or pachymeninges, lies directly beneath and is adherent to the skull. The other two membranes are referred to collectively as leptomeninges. Pia mater lies directly over brain tissue. Arachnoid, the middle layer, lies between the dura mater and the pia mater. The subarachnoid space, located between the arachnoid and the pia mater, is the conduit for CSF. By definition, meningitis refers to inflammation of the subarachnoid space or spinal fluid, whereas encephalitis is an inflammation of the brain itself. Since infectious microorganisms frequently are an underlying cause of these inflammatory processes, the terms meningitis and encephalitis frequently are used to denote an infectious process. The decision regarding the diagnosis of meningoencephalitis depends on radiographic, laboratory, and clinical information but would refer to inflammation of both tissue and fluid.
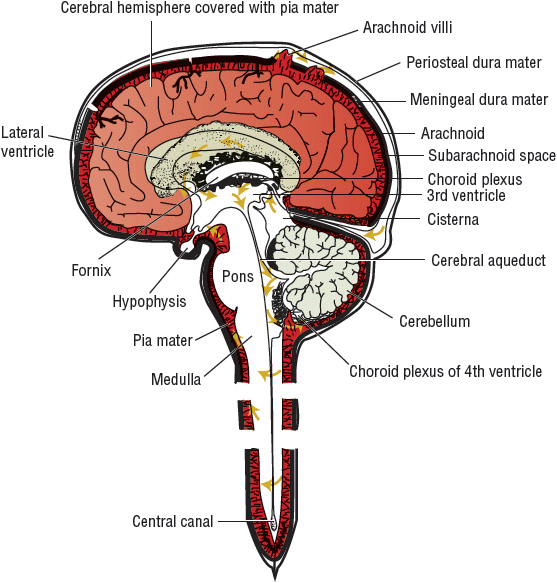
FIGURE 84-1 Diagram of the CNS.
Cerebrospinal Fluid
Approximately 85% of the CSF is produced within the third, fourth, and lateral ventricles by the choroid plexus (Fig. 84–1). CSF volume in the CNS is related to patient age: infants have approximately 40 to 60 mL of CSF, older children have 60 to 100 mL, and adults have 115 to 160 mL. Normally, CSF is produced at the rate of approximately 500 mL/day and flows unidirectionally downward through the spinal cord. The CSF is removed by the arachnoid villi and vertebral venous plexus located in the spinal cord and does not recommunicate with the point of production.11
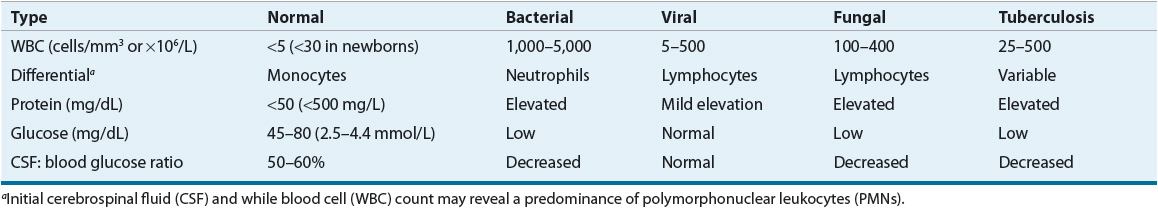
![]() The CSF normally is clear, with a protein content of less than 50 mg/dL (500 mg/L), a glucose concentration of approximately 50% to 60% of the simultaneous peripheral serum glucose concentration, and a pH of approximately 7.4. Also, it typically contains fewer than 5 white blood cells (WBCs)/mm3 (or fewer than 5 × 106/L), all of which should be lymphocytes (Table 84–1). As meninges become inflamed, the constituency of the CSF will change, and these changes can be used diagnostically as markers of infection.
The CSF normally is clear, with a protein content of less than 50 mg/dL (500 mg/L), a glucose concentration of approximately 50% to 60% of the simultaneous peripheral serum glucose concentration, and a pH of approximately 7.4. Also, it typically contains fewer than 5 white blood cells (WBCs)/mm3 (or fewer than 5 × 106/L), all of which should be lymphocytes (Table 84–1). As meninges become inflamed, the constituency of the CSF will change, and these changes can be used diagnostically as markers of infection.
Blood–Brain Barrier/Blood–CSF Barrier
Natural barriers to the exchange of drugs and endogenous compounds among the blood, brain, and CSF are the blood–brain barrier and the blood–CSF barrier (BCSFB) (Fig. 84–2). The blood–brain barrier consists of tightly joined capillary endothelial cells. Drug entry into brain tissue is accomplished by direct passage through the capillary endothelial cells and further penetration of the glial cells that envelop the capillary structure.11
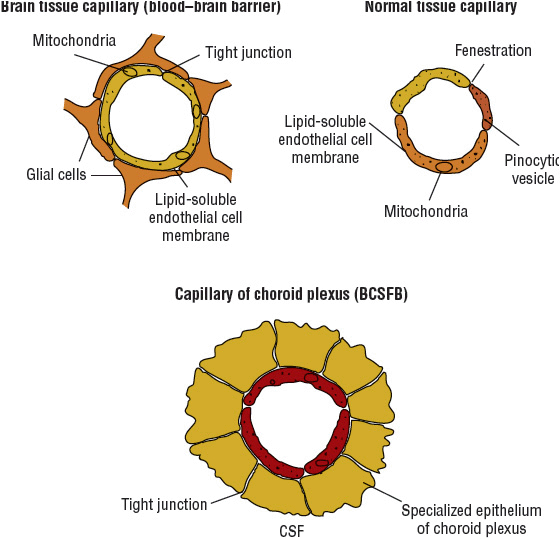
FIGURE 84-2 Schematic representation of a blood–cerebrospinal fluid barrier capillary, brain tissue capillary, and normal tissue capillary (below).
Passage of drugs into the CSF is controlled by the BCSFB. This barrier is created by ependymal cells of the choroid plexus, which function as an active transport system similar to the renal tubular epithelial cells. The inflammatory process associated with meningitis inhibits the active transport system of the choroid plexus.12 As in the active transport system in the kidney, the secretion of substances out of the choroid plexus also can be inhibited by the administration of probenecid.11
PATHOPHYSIOLOGY OF THE CNS INFECTION
The development of bacterial meningitis occurs following bacterial invasion of the host and CNS, bacterial multiplication with subsequent inflammation of the CNS, specifically the subarachnoid space and the ventricular space, pathophysiologic alterations owing to progressive inflammation, and the resulting neuronal damage.13 The critical first step in the acquisition of ABM is nasopharyngeal colonization of the host. Igs such as secretory IgA are found in high concentrations within nasopharyngeal secretions and work to inhibit bacterial colonization. However, the mucus barrier is deteriorated by IgA proteases secreted by the bacteria, which then extend pili that allow adherence to the host cell surface receptors. Bacterial pathogens attach themselves to nasopharyngeal epithelial cells and are phagocytized into the host’s bloodstream. After accessing the patient’s bloodstream, bacteria must overcome the host’s defense mechanisms. Commonly, CNS bacterial pathogens will produce an extensive polysaccharide capsule resistant to neutrophil phagocytosis and complement opsonization. H. influenzae, Escherichia coli, and N. meningitidis strains lacking polysaccharide capsules are unable to cause meningitis. Capsular polysaccharides activate the alternate complement pathway, which promotes phagocytosis and clearance of infecting pathogens. Patients unable to activate the alternative complement pathway, such as asplenic and sickle cell patients, are predisposed to bacterial infections caused by encapsulated microorganisms and therefore are at increased risk for meningitis.13
Although the exact site and mechanism of bacterial invasion into the CNS is unknown, studies suggest that invasion into the subarachnoid space occurs by continuous exposure of the CNS to large bacterial inocula. Bacteremia with inoculum densities of at least 103 colony-forming units (CFU)/mL (106 CFU/L) appears to be essential for subarachnoid space invasion.14 Although several sites of bacterial invasion have been theorized, the most plausible sites are the choroid plexus and/or the cerebral microvasculature. Host defense mechanisms within the subarachnoid space are inadequate to combat bacterial pathogens; therefore, bacteria replicate freely within the CSF until either overgrowth occurs or an effective antibiotic regimen is administered that terminates the process.
The effects of meningitis, namely, inflammation within the subarachnoid space and the ensuing neurologic damage, are not necessarily a direct result of the pathogens themselves. The neurologic sequelae occur due to the activation of the host’s inflammatory pathways, which is induced by the pathogens or their products. Bacterial cell lysis and subsequent death can result in the release of cell wall components, such as lipopolysaccharide (LPS), lipid A (endotoxin), lipoteichoic acid, teichoic acid, and peptidoglycan, depending on whether the pathogen is gram-positive or gram-negative (Fig. 84–3). These cell wall components cause capillary endothelial cells and CNS macrophages to release cytokines (interleukin 1 [IL-1] and tumor necrosis factor [TNF]) and other inflammatory mediators (IL-6, IL-8, platelet-activating factor [PAF], nitric oxide, arachidonic acid metabolites [e.g., prostaglandin and prostacycline], and macrophage-derived proteins). Proteolytic products and toxic oxygen radicals are released from the capillary endothelium, causing an alteration in the permeability of the blood–brain barrier. PAF activates the coagulation cascade, and arachidonic acid metabolites stimulate vasodilation. These events propagate other sequential events that lead to cerebral edema, elevated intracranial pressure (ICP), CSF pleocytosis, decreased cerebral blood flow (CBF), cerebral ischemia, and death.13,14
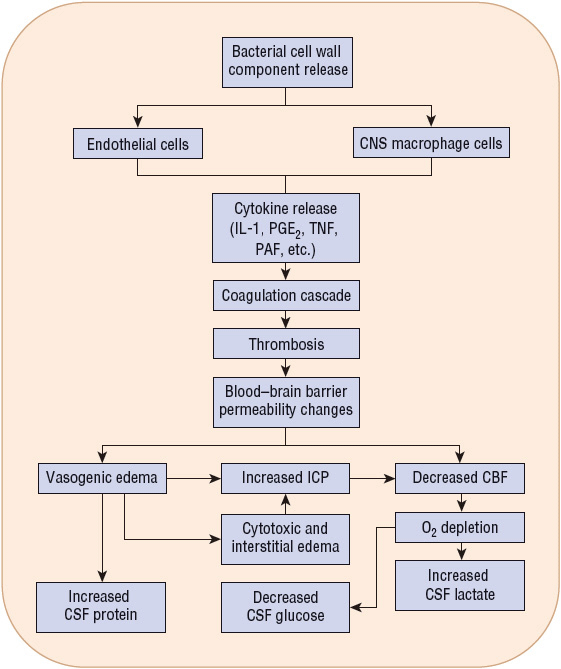
FIGURE 84-3 Hypothetical schema of pathophysiologic events that occur during bacterial meningitis. (IL-1, interleukin 1; TNF, tumor necrosis factor; PAF, platelet-activating factor; CBF, cerebral blood flow; CSF, cerebrospinal fluid; PGE2, prostaglandin E2; ICP, intracranial pressure.)
Clinical Controversy…
CLINICAL PRESENTATION AND DIAGNOSIS
Clinical presentation varies with age, and, generally, the younger the patient, the more atypical and the less pronounced is the clinical picture. Patients may receive antibiotics before a diagnosis of meningitis is made, delaying presentation to the hospital. Prior antibiotic therapy may cause the Gram stain and CSF culture to be negative, but the antibiotic therapy rarely affects CSF protein or glucose.
Signs and Symptoms
![]() Classic signs and symptoms include fever, nuchal rigidity, altered mental status (the classic triad), chills, vomiting, photophobia, and severe headache; Kernig’s and Brudzinski’s signs may also be present but are poorly sensitive and frequently are absent in children (Figs. 84-4 and 84-5).15 Clinical signs and symptoms in young children may include bulging fontanelle, apneas, purpuric rash, irritability, refusal to eat, and convulsions in addition to those just mentioned.15 Almost all patients have at least two of the following symptoms: fever, nuchal rigidity, headache, and altered mental status.4,16 Purpuric and petechial skin lesions typically indicate meningococcal involvement, although the lesions may be present with H. influenzae meningitis. Rashes rarely occur with pneumococcal meningitis.17
Classic signs and symptoms include fever, nuchal rigidity, altered mental status (the classic triad), chills, vomiting, photophobia, and severe headache; Kernig’s and Brudzinski’s signs may also be present but are poorly sensitive and frequently are absent in children (Figs. 84-4 and 84-5).15 Clinical signs and symptoms in young children may include bulging fontanelle, apneas, purpuric rash, irritability, refusal to eat, and convulsions in addition to those just mentioned.15 Almost all patients have at least two of the following symptoms: fever, nuchal rigidity, headache, and altered mental status.4,16 Purpuric and petechial skin lesions typically indicate meningococcal involvement, although the lesions may be present with H. influenzae meningitis. Rashes rarely occur with pneumococcal meningitis.17
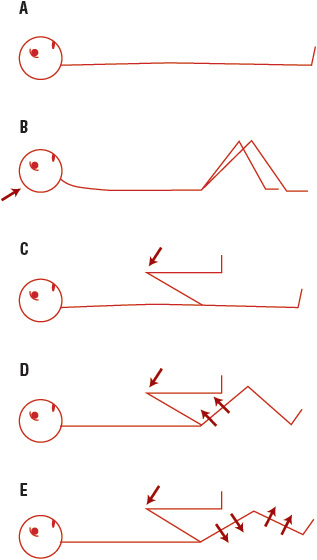
FIGURE 84-4A, B. Brudzinski’s neck signs. Hip and knee flexion occurs as a result of flexion of the neck (B). C–E. Brudzinski’s leg signs. C. Patient’s leg is flexed by examiner (arrow). D. The contralateral leg begins to flex—identical contralateral sign (arrows). E. The contralateral leg now begins to extend spontaneously, resembling a little kick (arrows).
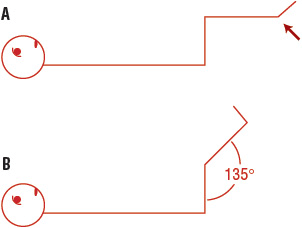
FIGURE 84-5 Kernig’s sign. A. Knees are raised to form a 90° angle relative to the trunk, and the examiner attempts to extend the knees. B. Once the knee angle reaches approximately 135°, contracture or extensor spasm occurs.
Waterhouse-Friderichsen syndrome, a rapid eruption of multiple hemorrhagic lesions associated with a shock-like state, is associated with meningococcal meningitis. Both H. influenzae meningitis and meningococcal meningitis can cause involvement of the joints during the illness. History of head trauma with or without skull fracture or presence of a chronically draining ear is associated with pneumococcal involvement.
Bacterial Meningitis Score
Bacterial Meningitis Score is a validated clinical decision tool aimed to identify children older than 2 months with CSF pleocytosis who are at low risk of ABM. This tool incorporates clinical features such as positive CSF Gram stain, presence of seizure, serum absolute neutrophil count ≥10,000 cells/mm3 (≥10 × 109/L), CSF protein ≥80 mg/dL (≥800 mg/L), and CSF neutrophil count ≥1,000 cells/mm3 (≥1 × 109/L). Treatment is recommended when one or more criteria are present. Certain pediatric patients are excluded including those with purpura, CSF shunt, recent neurosurgery, and Lyme’s disease (LD) and those who received oral or IV antibiotics within 72 hours. This scoring tool was validated in several studies showing high accuracy in excluding ABM. One meta-analysis of eight validation studies between 2002 and 2012 (5,312 pediatric patients) showed the tool to be highly accurate, with combined sensitivity of 99.3%, specificity of 62.1%, and negative predictive value of 99.7%.18
Laboratory Tests
Several tubes of CSF are collected via lumbar puncture for chemistry, microbiology, and hematology tests. Theoretically, the first tube has a higher likelihood of being contaminated with both blood and bacteria during the puncture, although the total volume is more important in practice than the tube cultured. CSF should not be refrigerated or stored on ice.
Analysis of CSF chemistries typically includes measurement of glucose and total protein concentrations. An elevated CSF protein of ≥50 mg/dL (≥500 mg/L) and a CSF glucose concentration of less than 50% of the simultaneously obtained peripheral value suggest bacterial meningitis (see Table 84–1).
The values for CSF glucose, protein, and WBC concentrations found with bacterial meningitis overlap significantly with those for viral, tuberculous, and fungal meningitis (see Table 84–1). Therefore, CSF WBC counts and CSF glucose and protein concentrations cannot always distinguish the different etiologies of meningitis.
Other Diagnostic Tests19–22
In patients presenting with new-onset seizures, signs of space-occupying lesions or moderate to severe impairment of consciousness, cranial imaging via magnetic resonance imaging (MRI), or cranial computed tomography (CT) should precede a lumbar puncture. MRI is generally preferred as it more clearly identifies areas of cerebral edemas. In these instances, the withdrawal of CSF fluid from a lumbar puncture reduces counterpressure that may result in compression of the brain from above with risk of brain herniation complicating the clinical course. Neuroimaging should not however delay initiation of antibiotic therapy as doing so can result in a poor outcome in this disease.23,24 MRI is the preferred modality for the diagnosis of encephalitis due to higher specificity and sensitivity (A-I) than CT (B-III) (see Table 84–2 footnotes for rating scale of evidence).
Blood and other specimens should be cultured according to clinical judgment because meningitis frequently can arise via hematogenous dissemination or can be associated with infections at other sites. A minimum of 20 mL of blood in each of two to three separate cultures per each 24-hour period is necessary for the detection of most bacteremia.
![]() Gram stain and culture of the CSF are the most important laboratory tests performed for bacterial meningitis. The Gram stain continues to be the most rapid and accurate method of presumptively diagnosing ABM. When performed before antibiotic therapy is initiated, Gram stain is both rapid and sensitive and can confirm the diagnosis of bacterial meningitis in 75% to 90% of cases. The sensitivity of the Gram stain decreases to 40% to 60% in patients who received prior antibiotic therapy. Culture is required to differentiate the various bacterial etiologies.
Gram stain and culture of the CSF are the most important laboratory tests performed for bacterial meningitis. The Gram stain continues to be the most rapid and accurate method of presumptively diagnosing ABM. When performed before antibiotic therapy is initiated, Gram stain is both rapid and sensitive and can confirm the diagnosis of bacterial meningitis in 75% to 90% of cases. The sensitivity of the Gram stain decreases to 40% to 60% in patients who received prior antibiotic therapy. Culture is required to differentiate the various bacterial etiologies.
Polymerase chain reaction (PCR) techniques can be used to diagnose meningitis caused by N. meningitidis, S. pneumoniae, and Hib. PCR is considered to be highly sensitive and specific, but expense and availability can be limiting. Currently, no U.S. FDA-approved testing is available.
Latex fixation, latex coagglutination, and enzyme immunoassay (EIA) tests provide for the rapid identification of several bacterial causes of meningitis, including S. pneumoniae, N. meningitidis, and Hib. Rapid-identification latex tests work by bringing potential capsular antigens of the pathogen causing meningitis in contact with a specific antibody, causing an antigen–antibody reaction. This capsular antigen–antibody reaction can be observed visually and quickly without waiting for culture results. The sensitivity and specificity of latex fixation and coagglutination tests can vary with the manufacturer of the antibody, density of the antigen present in the CSF, and pathogen being tested. Latex agglutination is most useful for patients who have been treated with antimicrobials and whose CSF Gram stain and culture are negative (B-III).
Diagnosis of tuberculosis meningitis employs acid-fast staining, culture, and PCR of the CSF. PCR testing of the CSF is the preferred method of diagnosing most viral meningitis infections (A-III). The standard diagnostic tests for fungal meningitis include culture, direct microscopic examination of stained and unstained specimens of CSF, antigen detection of cryptococcal or histoplasmal antigens, and antibody assay of serum and/or CSF.
TREATMENT
Desired Outcome
![]() Goals for the treatment of CNS infections should include eradication of infection, amelioration of signs and symptoms, preventing morbidity and mortality, initiating appropriate antimicrobials, providing supportive care, and preventing disease through timely introduction of vaccination and chemoprophylaxis. Understanding antibiotic selection and the issues surrounding antibiotic penetration will assist in meeting the goals of treatment.
Goals for the treatment of CNS infections should include eradication of infection, amelioration of signs and symptoms, preventing morbidity and mortality, initiating appropriate antimicrobials, providing supportive care, and preventing disease through timely introduction of vaccination and chemoprophylaxis. Understanding antibiotic selection and the issues surrounding antibiotic penetration will assist in meeting the goals of treatment.
General Approach to Treatment and Nonpharmacologic and Supportive Therapy
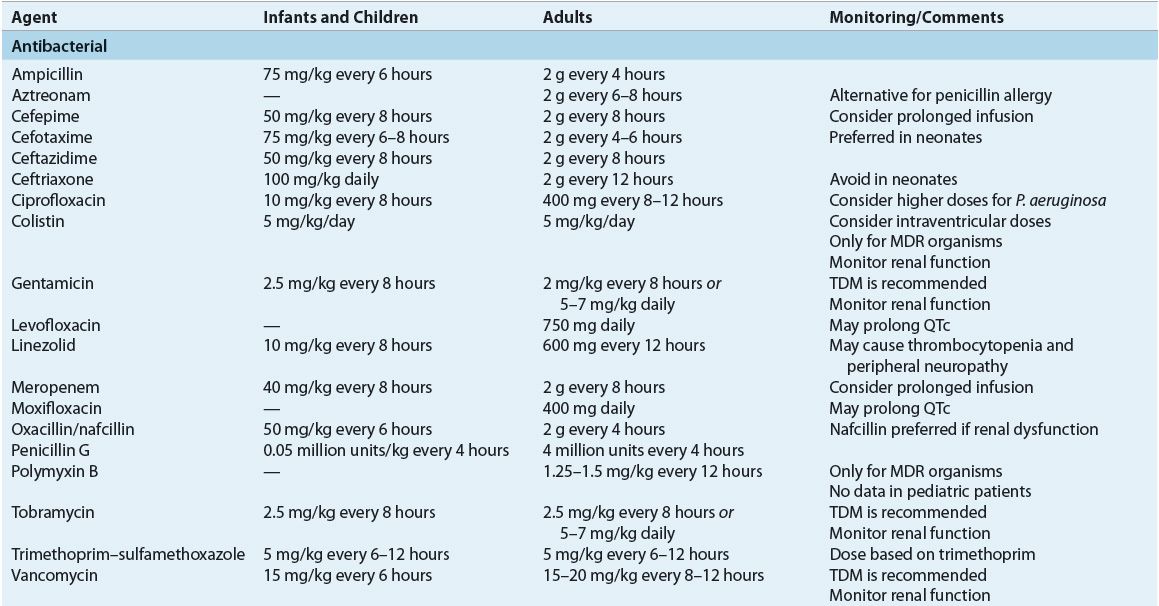
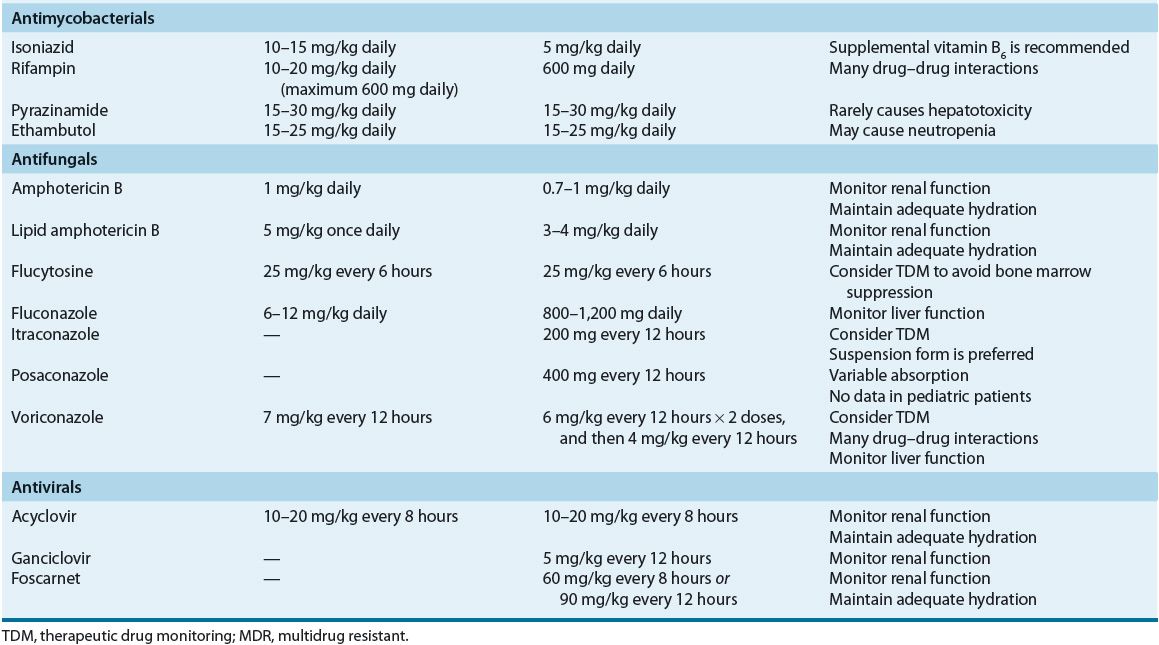
This section discusses issues surrounding the approach to treatment, such as antibiotic penetration within the CNS, duration of antibiotic therapy, and supportive treatments. Until a pathogen is identified, prompt empirical antibiotic coverage is often needed. ![]() Based on the patient’s profile (i.e., allergies, age, and concurrent medical conditions), extent of antibiotic CNS penetration,25 and spectrum of activity, appropriate recommendations can be made, and therapy should last at least 48 to 72 hours or until the diagnosis of bacterial meningitis can be ruled out (Tables 84–2 and 84-3).
Based on the patient’s profile (i.e., allergies, age, and concurrent medical conditions), extent of antibiotic CNS penetration,25 and spectrum of activity, appropriate recommendations can be made, and therapy should last at least 48 to 72 hours or until the diagnosis of bacterial meningitis can be ruled out (Tables 84–2 and 84-3). ![]() The first dose of antibiotics should not be withheld, even when lumbar puncture is delayed or neuroimaging is being performed. Changes in the CSF after antibiotic administration usually take 12 to 24 hours. Continued therapy should be based on the assessment of clinical improvement, cultures, and susceptibility testing results. Once a pathogen is identified, antibiotic therapy should be tailored to the specific pathogen (Tables 84–4 and 84-5). Throughout the course of treatment, efficacy parameters, such as signs and symptoms, microbiologic findings, and CSF examination, should be followed to evaluate the success of meeting the desired outcomes.
The first dose of antibiotics should not be withheld, even when lumbar puncture is delayed or neuroimaging is being performed. Changes in the CSF after antibiotic administration usually take 12 to 24 hours. Continued therapy should be based on the assessment of clinical improvement, cultures, and susceptibility testing results. Once a pathogen is identified, antibiotic therapy should be tailored to the specific pathogen (Tables 84–4 and 84-5). Throughout the course of treatment, efficacy parameters, such as signs and symptoms, microbiologic findings, and CSF examination, should be followed to evaluate the success of meeting the desired outcomes.
TABLE 84-3 Penetration of Antimicrobial Agents into the CSFa, 25
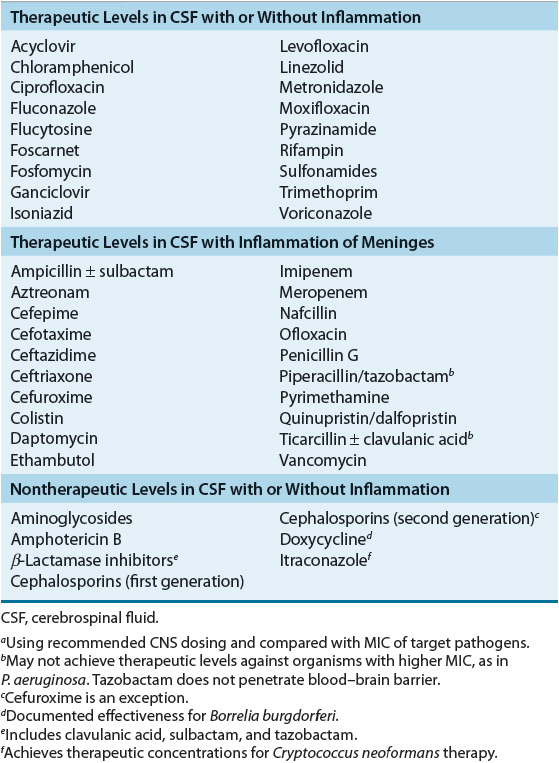
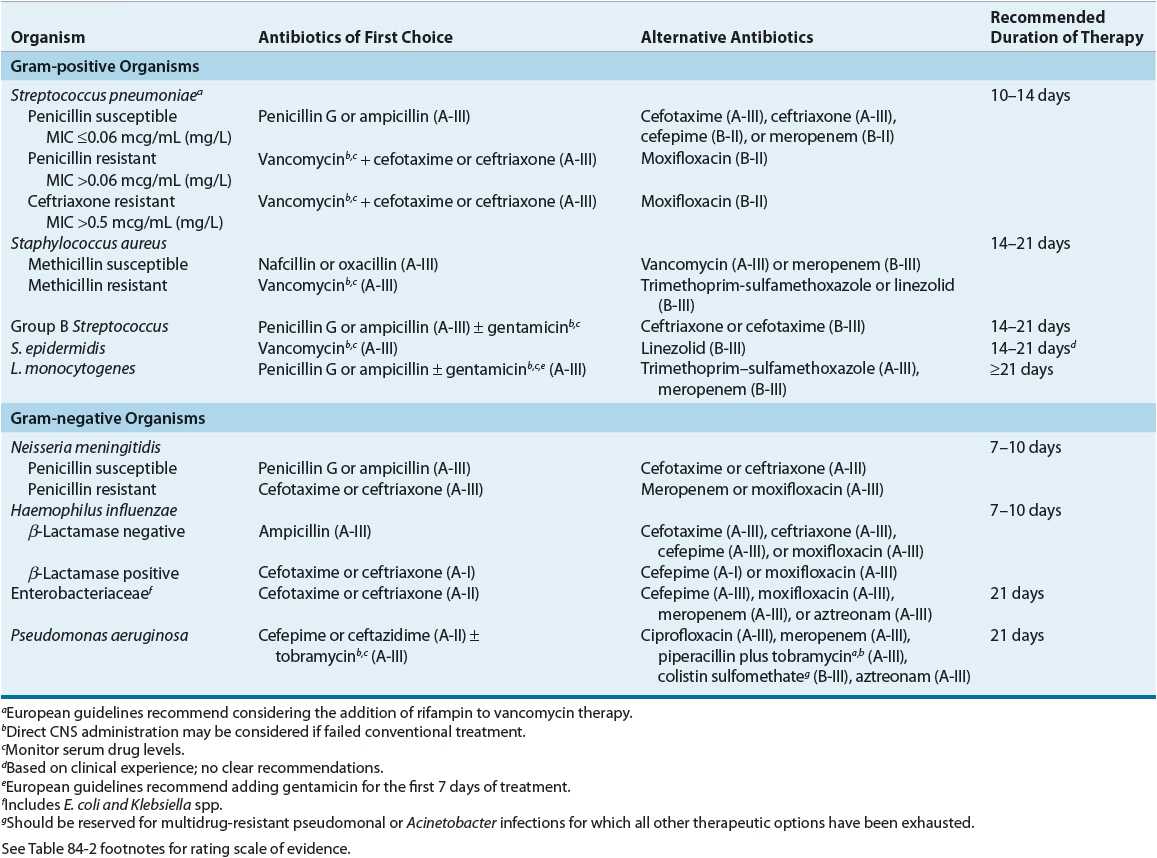
TABLE 84-4 Antimicrobial Agents of First Choice and Alternative Choice in the Treatment of Meningitis Caused by Gram-Positive and Gram-Negative Microorganisms16,19,20
Supportive care, particularly early in the course of treatment, is critically important. Administration of fluids, electrolytes, antipyretics, and analgesics is indicated for patients presenting with ABM. Additionally, venous thromboembolism prophylaxis and ICP monitoring are often needed. Patients may require the administration of osmotic diuretics such as mannitol 25% or hypertonic 3% saline to maintain an ICP of <15 mm Hg (<2 kPa) and a cerebral perfusion pressure of ≥60 mm Hg (≥8 kPa). Other supportive care measures may include respiratory and circulatory support, GI care, and maintaining normal body temperature. Although supportive care is important initially, appropriate antibiotic therapy (empirical or definitive) should be started as soon as possible.23,24
![]() Several factors influence the transfer of antibiotic from capillary blood into the CNS, including inflammation of the meninges, which increases antibiotic penetration through damage to tight junctions between capillary endothelial cells and decreases the activity of an energy-dependent efflux pump in the choroid plexus responsible for movement of penicillins and, to a much lesser extent, fluoroquinolones and aminoglycosides (see Table 84–3). Antibiotics having low molecular weights are passed more easily through biologic barriers than compounds of higher molecular weight. Only antibiotics that are nonionized at physiologic or pathologic pH are capable of diffusion. Highly lipid-soluble compounds penetrate more readily than water-soluble compounds. Antibiotics not extensively bound to plasma proteins provide a larger free fraction of drug capable of passing into the CSF. Passage of large, polar antibiotics into the CSF may be assisted, however, by a carrier transport system. Antibiotic dosages in the treatment of CNS infections must be optimized to ensure adequate penetration to the site of infection.
Several factors influence the transfer of antibiotic from capillary blood into the CNS, including inflammation of the meninges, which increases antibiotic penetration through damage to tight junctions between capillary endothelial cells and decreases the activity of an energy-dependent efflux pump in the choroid plexus responsible for movement of penicillins and, to a much lesser extent, fluoroquinolones and aminoglycosides (see Table 84–3). Antibiotics having low molecular weights are passed more easily through biologic barriers than compounds of higher molecular weight. Only antibiotics that are nonionized at physiologic or pathologic pH are capable of diffusion. Highly lipid-soluble compounds penetrate more readily than water-soluble compounds. Antibiotics not extensively bound to plasma proteins provide a larger free fraction of drug capable of passing into the CSF. Passage of large, polar antibiotics into the CSF may be assisted, however, by a carrier transport system. Antibiotic dosages in the treatment of CNS infections must be optimized to ensure adequate penetration to the site of infection.
Problems of CSF penetration were traditionally overcome by direct instillation of antibiotics intrathecally, intracisternally, or intraventricularly. Advantages of direct instillation, however, must be weighed against the risks of invasive CNS procedures. Intrathecal administration of antibiotics is unlikely to produce therapeutic concentrations in the ventricles possibly owing to the unidirectional flow of CSF.26 Although intraventricular administration from a therapeutic standpoint may be preferred over intrathecal administration, the former requires neurosurgical placement of a subcutaneous reservoir. Intraventricular delivery may be necessary in patients who have shunt infections that are difficult to eradicate or who cannot undergo surgical interventions (A-III).19 The antimicrobial agents often utilized for ABM treatment have adequate CSF penetration, which has limited the need for direct CNS instillation of antibiotics. The European guidelines for meningitis treatment recommend considering the use of intrathecal or intraventricular antibiotics only in patients who fail conventional treatment.20
![]() Although the length of treatment for bacterial meningitis generally is based on the causative organism, there is no universally accepted standard (Table 84–4). Meningitis caused by S. pneumoniae has been treated successfully with 10 to 14 days of antibiotic therapy. Meningitis caused by N. meningitidis or H. influenzae usually can be treated with a 7-day course of antibiotics. In contrast, a longer duration (≥21 days) has been recommended for patients with L. monocytogenes, gram-negative or pseudomonal meningitis (A-III). Therapy should be individualized, and some patients may require enduring courses.19,20
Although the length of treatment for bacterial meningitis generally is based on the causative organism, there is no universally accepted standard (Table 84–4). Meningitis caused by S. pneumoniae has been treated successfully with 10 to 14 days of antibiotic therapy. Meningitis caused by N. meningitidis or H. influenzae usually can be treated with a 7-day course of antibiotics. In contrast, a longer duration (≥21 days) has been recommended for patients with L. monocytogenes, gram-negative or pseudomonal meningitis (A-III). Therapy should be individualized, and some patients may require enduring courses.19,20
Clinical Controversy…
Causative Organisms
S. pneumoniae (Pneumococcus or Diplococcus)
![]() S. pneumoniae is the leading cause of meningitis in patients ≥2 months of age in the United States. Overall case-fatality rate is estimated to be 18%. Despite the decline in rates of pneumococcal meningitis since the introduction of PCV7 vaccination in 2000, case-fatality rate did not significantly change from pre-PCV7 era.8 Approximately 50% of cases are secondary infections resulting from primary infections of parameningeal foci, such as the ear or paranasal sinuses. Pneumonia, endocarditis, CSF leak secondary to head trauma, splenectomy, alcoholism, sickle cell disease, and bone marrow transplantation may predispose the patient to the development of pneumococcal meningitis.
S. pneumoniae is the leading cause of meningitis in patients ≥2 months of age in the United States. Overall case-fatality rate is estimated to be 18%. Despite the decline in rates of pneumococcal meningitis since the introduction of PCV7 vaccination in 2000, case-fatality rate did not significantly change from pre-PCV7 era.8 Approximately 50% of cases are secondary infections resulting from primary infections of parameningeal foci, such as the ear or paranasal sinuses. Pneumonia, endocarditis, CSF leak secondary to head trauma, splenectomy, alcoholism, sickle cell disease, and bone marrow transplantation may predispose the patient to the development of pneumococcal meningitis.
Neurologic complications, such as coma, hearing impairment, and seizures, are common with pneumococcal meningitis. The prognosis of pneumococcal meningitis depends on a variety of factors, including chronic comorbidities, low Glasgow Coma Scale Score, focal neurologic deficits on admission, low CSF leukocyte count, pneumonia, bacteremia, and intracranial and systemic complications.27
Based on resistance patterns and the fact that sufficient CSF concentrations of penicillin are difficult to achieve with standard IV doses, penicillin should not be used as empirical therapy if S. pneumoniae is a suspected pathogen. Furthermore, appropriate Clinical Laboratory Standards Institute (CLSI)–approved testing of all CSF isolates for penicillin resistance is recommended. Ceftriaxone and cefotaxime have served as alternatives to penicillin in the treatment of penicillin-resistant pneumococci. Of note, higher cephalosporin minimum inhibitory concentration (MIC) and higher cephalosporin resistance rates were shown in penicillin-resistant isolates.28 Therapeutic approaches to cephalosporin-resistant pneumococcus include the addition of vancomycin and rifampin. However, only data from animal and experimental trials supporting the use of rifampin are available.29,30 ![]() Therefore, the combination of vancomycin and ceftriaxone has been suggested as empirical treatment until the results of antimicrobial susceptibility testing are available (A-III). Vancomycin should not be used alone even for highly penicillin- and cephalosporin-resistant strains (A-III).19,20 Some pneumococcal strains exhibit tolerance to vancomycin and were linked to increased meningitis mortality.31,32
Therefore, the combination of vancomycin and ceftriaxone has been suggested as empirical treatment until the results of antimicrobial susceptibility testing are available (A-III). Vancomycin should not be used alone even for highly penicillin- and cephalosporin-resistant strains (A-III).19,20 Some pneumococcal strains exhibit tolerance to vancomycin and were linked to increased meningitis mortality.31,32
Based on concern about the limited therapeutic options for penicillin- and cephalosporin-resistant pneumococcal meningitis, newer agents have been evaluated. Meropenem is approved by the U.S. FDA for the treatment of bacterial meningitis in children aged 3 months and older and has shown similar clinical and microbiologic efficacy to cefotaxime or ceftriaxone. It is currently recommended as an alternative to a third-generation cephalosporin in penicillin-nonsusceptible isolates (B-II). Some caution is warranted with the use of imipenem for CNS infections because of the possibility of drug-induced seizures, especially when not properly dose adjusted for declining renal function. Of note, seizures may be caused by meningitis itself or by imipenem, and the cause is difficult to differentiate. The newer fluoroquinolones represent another therapeutic option owing to favorable activity against multidrug-resistant pneumococci and good penetration into the CSF (B-II).33
IV linezolid and daptomycin have emerged as therapeutic options for treating multidrug-resistant gram-positive infections. Linezolid in combination with ceftriaxone has been used to treat a limited number of cases of pneumococcal meningitis with outcomes similar to standard treatment.34 The penetration of daptomycin in the CSF was approximately 6% following a 15 mg/kg bolus achieving maximum concentration approximately 4 hours after the dose in a rabbit meningitis model. The 15 mg/kg dose produces similar serum concentrations in rabbits as the 6 mg/kg dose in humans. In this study, daptomycin was able to clear both the penicillin-resistant and the quinolone-resistant pneumococci from the CSF more rapidly than the standard regimen of vancomycin and ceftriaxone.35 Additionally, daptomycin may reduce the inflammatory response caused by cell wall components in pneumococcal meningitis compared with ceftriaxone in animal models.36
Pneumococcal vaccines help in reducing the risk of invasive pneumococcal disease. Virtually all serotypes of S. pneumoniae exhibiting intermediate or complete resistance to penicillin are found in the 23-valent pneumococcal polysaccharide vaccine (PPV23) (Pneumovax 23®). Due to low vaccination rates among people 65 years of age and older, the U.S. Centers for Disease Control and Prevention (CDC) issued stronger recommendations for the use of the pneumococcal polysaccharide vaccine, calling for vaccination of the following high-risk groups: persons over the age of 65 years; persons aged 2 to 64 years who have a chronic illness, who live in high-risk environments (e.g., Alaskan Natives and residents of long-term care facilities), and who lack a functioning spleen (e.g., sickle cell disease and splenectomy); and immunocompromised persons over the age of 2 years, including those with human immunodeficiency virus (HIV) infection. Additionally, the question of whether or not college students living in dormitories, a possible high-risk environment, should be vaccinated remains debatable. Unfortunately, variability in the host’s ability to mount an immune response to the vaccine limits its usefulness for penicillin-resistant pneumococci in children younger than 2 years of age and in immunocompromised adults.
In 2000, a heptavalent pneumococcal protein-conjugate vaccine (PCV7) (Prevnar®) was approved for use in children 2 months of age and older. Use of the vaccine has significantly reduced invasive pneumococcal infections, including sepsis and meningitis, as well as possible cost savings.9,37 Moreover, the vaccine is safe and effective in low-birth-weight and preterm infants.38 In the decade following the introduction of PCV7, rate of invasive disease caused by non-PCV7 strains increased considerably, especially serotype 19A.9 This led to the development of a newer vaccine with expanded coverage. Ultimately, the FDA approved a 13-valent pneumococcal conjugate vaccine (PCV13) (Prevnar 13®) in 2010 for childhood vaccination. Current recommendations are for all healthy infants younger than 2 years of age to be immunized with the PCV13 at 2, 4, 6, and 12 to 15 months. The CDC has issued a recommendation that all persons with cochlear implants receive age-appropriate vaccination with the pneumococcal conjugate vaccine, pneumococcal polysaccharide vaccine, or both.39 In 2011, the FDA approved the use of PCV13 in adults 50 years and older as PCV13 was shown to produce antibody levels that are either comparable to or higher than the levels achieved by PPV23, for the 13 serotypes included in PCV13. Final recommendations from the CDC regarding the routine use of PCV13 in adults will be forthcoming.
N. meningitidis (Meningococcus)
![]() N. meningitidis is a leading cause of bacterial meningitis among children and young adults in the United States and around the world.8,40 The source of infection usually is an asymptomatic carrier. Five of the 13 serogroups of N. meningitidis (A, B, C, Y, and W-135) are primarily responsible. Clusters of meningococcal disease, defined as two or more cases of the same serogroup that are closer in time and space than expected for the population or group under observation, generally are associated with crowding as in schools, dormitories, and military barracks.41 Although some of these clusters have been due to serogroup B, the majority has been due to serogroup C. Serogroup A, although associated with meningococcal outbreaks in Africa and Asia, is a rare cause of disease in the United States. Serogroup Y, although frequently associated with pneumonia, is emerging as an important cause of invasive meningococcal disease in select areas.42 Overall, N. meningitidis accounted for 13.9% of all meningitis cases in the United States during 2003 to 2007, most cases in persons aged 2 to 18 years. According to recent estimates, the case-fatality rate is approximately 10%.8
N. meningitidis is a leading cause of bacterial meningitis among children and young adults in the United States and around the world.8,40 The source of infection usually is an asymptomatic carrier. Five of the 13 serogroups of N. meningitidis (A, B, C, Y, and W-135) are primarily responsible. Clusters of meningococcal disease, defined as two or more cases of the same serogroup that are closer in time and space than expected for the population or group under observation, generally are associated with crowding as in schools, dormitories, and military barracks.41 Although some of these clusters have been due to serogroup B, the majority has been due to serogroup C. Serogroup A, although associated with meningococcal outbreaks in Africa and Asia, is a rare cause of disease in the United States. Serogroup Y, although frequently associated with pneumonia, is emerging as an important cause of invasive meningococcal disease in select areas.42 Overall, N. meningitidis accounted for 13.9% of all meningitis cases in the United States during 2003 to 2007, most cases in persons aged 2 to 18 years. According to recent estimates, the case-fatality rate is approximately 10%.8
Initially, patients are colonized and, at some point, develop bacteremia, which most likely occurs prior to hospital admission. Meningitis occurs after the bacteria seed into the meninges. After the acute phase of meningitis has resolved, there is a unique immune reaction that distinguishes meningococcal meningitis from other bacterial causes. ![]() The patient develops a characteristic immunologic reaction of fever, arthritis (usually involving large joints), and pericarditis approximately 10 to 14 days after the onset of disease and despite successful treatment. At this time, examination of the synovial fluid reveals a large number of polymorphonuclear cells, elevated protein concentrations, normal glucose concentrations, and sterile cultures. The reaction may last a week or longer, and no additional antibiotic therapy is required; however, patients may benefit from nonsteroidal antiinflammatory agents and supportive care.17,43
The patient develops a characteristic immunologic reaction of fever, arthritis (usually involving large joints), and pericarditis approximately 10 to 14 days after the onset of disease and despite successful treatment. At this time, examination of the synovial fluid reveals a large number of polymorphonuclear cells, elevated protein concentrations, normal glucose concentrations, and sterile cultures. The reaction may last a week or longer, and no additional antibiotic therapy is required; however, patients may benefit from nonsteroidal antiinflammatory agents and supportive care.17,43
Seizures and coma are uncommon with meningococcal meningitis. Patients may behave aggressively and often are maniacal. They may develop deafness and transiently impaired ocular movements. Deafness unilaterally or, more commonly, bilaterally may develop early or late in the disease course. Hearing loss secondary to sensory nerve damage (sensorineural hearing) is usually permanent, whereas conductive hearing impairment, such as damage to the tympanic membrane, is often reversible.17,43
The presence of petechiae may be the primary clue that the underlying pathogen is N. meningitidis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree