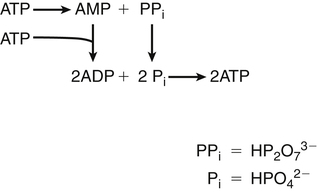
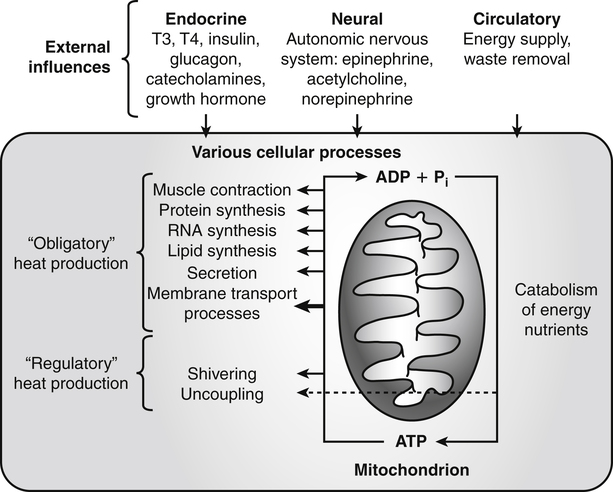
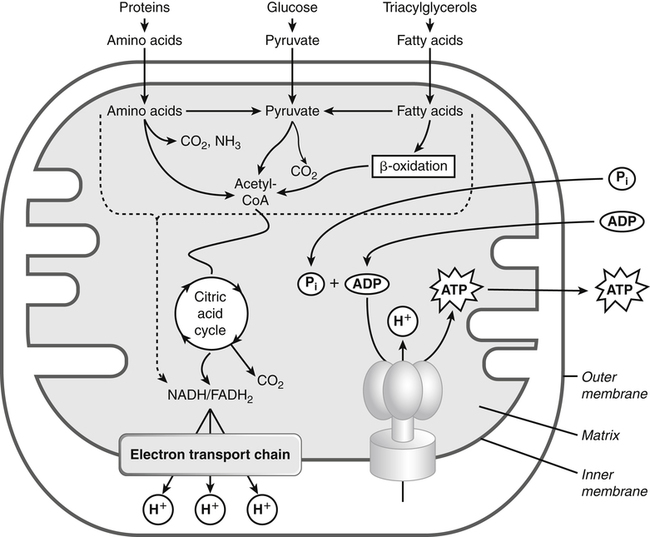
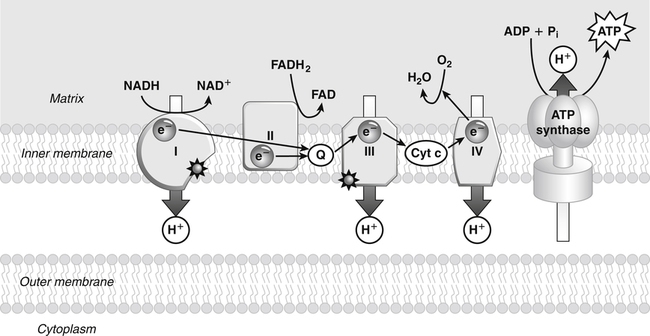
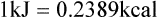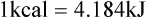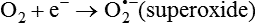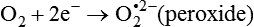Darlene E. Berryman, PhD, RD and Matthew W. Hulver, PhD Total-body energy utilization is the sum of the collective energy-utilizing and energy-producing reactions occurring within individual cells throughout the body. Both energy in foods and energy expenditure by the body are typically measured in calories or joules. The various metabolic reactions that serve to sustain life are fueled by the energy released from the biological oxidation of energy-yielding nutrients in the food we consume. A fraction of the energy released from biological oxidation processes is captured in high-energy bonds in molecules, namely in the phosphate−phosphate bonds of adenosine 5′-triphosphate (ATP), which is the main energy currency of the cell. ATP is then used to fuel the various biochemical processes within cells that support basal metabolism, growth, and other essential functions. Animal cells obtain energy by oxidizing food molecules. The oxidation of fuel molecules in the body is referred to as catabolism. The metabolic reactions that result in breakdown of the fuel molecules are not completely efficient and result in loss of some of the inherent energy in the fuel molecule as heat. However, a substantial portion of the energy in the fuel molecules is conserved as either ATP or the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH). Whereas ATP is the main source of energy for driving most cellular functions, NADPH is used mainly to drive reactions involved in the synthesis of fatty acids and sterols and a few other reactions such as reduction of oxidized glutathione (see Chapters 12, 16, and 17). Thus ATP and NADPH serve a unique role in coupling the energy-yielding processes of catabolism to the energy-consuming reactions of cellular work. ATP is the main energy source for the majority of cellular functions, being hydrolyzed to ADP when it is consumed. ATP is often considered the energy currency of the cell, with the metabolic unit of exchange being the ATP equivalent. An ATP equivalent is defined as the conversion of ATP to ADP (or ADP to ATP). ATP is generated by energy-releasing (exothermic) processes but consumed by energy-requiring (endothermic) processes. Some reactions are driven by hydrolysis of GTP, uridine 5′-triphosphate (UTP), or creatine phosphate, but these are considered equivalent to ATP expenditure because rephosphorylation of GDP, UDP, or creatine may be accomplished by reactions coupled to the conversion of ATP to ADP. Some reactions require more energy than can be obtained from ATP hydrolysis to ADP + inorganic phosphate (Pi) and involve hydrolysis of ATP to adenosine 5′-monophosphate (AMP) + pyrophosphate (PPi), which is further hydrolyzed to 2 Pi. This is considered equivalent to expenditure of 2 ATPs because expenditure of 2 ATPs is required for ATP resynthesis from AMP as shown in Figure 21-1. In addition to serving as the major energy currency of the cell, ATP also serves as an important allosteric effector in the regulation of metabolism. Its concentration relative to those of ADP and AMP is an index of the energy status of the cell and determines the rates of regulatory enzymes situated at key points in metabolism. AMP-activated protein kinase (AMPK) is an important sensor of intracellular AMP/ATP ratios that exerts regulatory effects on multiple ATP-producing and ATP-consuming pathways (see Figure 19-1 in Chapter 19). All living cells produce heat as a by-product of metabolism. Heat production within cells of an organism in the resting, postabsorptive state has two essential components: (1) obligatory and (2) regulatory (Figure 21-2). Obligatory heat production is the heat released during anabolic and catabolic reactions responsible for all cellular processes, primarily through the utilization and resynthesis of ATP molecules. At rest, the majority of the energy expended by a cell is being used to drive various molecular transport mechanisms, many of which are responsible for maintaining essential electrochemical gradients across membranes. For example, it has been estimated that the Na+,K+-ATPase pump reaction, which couples ATP hydrolysis to transport of K+ into cells and movement of Na+ out of cells, accounts for 20% to 40% of whole-body resting energy expenditure (McBride and Kelly, 1990). In highly metabolically active cell types, such as hepatocytes (liver cells), proton (H+) pumping across the mitochondrial membrane may represent up to 30% of the energy used by the cell. At rest, other processes that contribute substantially to energy needs include reactions responsible for macromolecular synthesis and degradation (e.g., protein and phospholipid turnover) and substrate cycling (discussed later in this chapter). Regulatory heat production is important for homeothermic organisms (i.e., those that maintain a constant body temperature). Regulatory heat production is needed to maintain a constant body temperature in the face of fluctuating environmental temperature. Shivering, which is triggered in response to cold exposure, can involve a substantial increase in skeletal muscle contraction, which results in a significant increase in ATP turnover and subsequently increased heat production. Heat generated through uncoupling mechanisms (discussed in a subsequent section of this chapter) can also contribute significantly to regulatory heat production. Conversely, exposure to increased environmental temperature stimulates sweat production, which helps cool the body through the heat loss associated with evaporation (see Chapter 35). The processes of modulating heat production and loss confer tremendous flexibility to many species so that they may survive in many different environmental conditions. Energy for the synthesis of ATP within cells comes mainly from the oxidation of energy-yielding molecules, which include predominantly carbohydrate and fat but also protein and alcohol (Flatt, 1985). The complete metabolic oxidation of fuel molecules to CO2 and H2O occurs with a similar stoichiometry to that for combustion of the fuels in a flame, but the processes involved are quite different. As summarized in Figure 21-3, catabolism of fuel molecules involves the oxidation of the fuel molecules coupled to the reduction of other molecules such as NAD(P) and FAD. Some ATP equivalents are formed by substrate-level phosphorylation (e.g., in two steps of glycolysis and in the citric acid cycle). CO2 is formed primarily from oxidation of acetyl-CoA by the citric acid cycle with H2O as the source of the additional oxygen needed to convert the acetyl moiety to CO2. However, most ATP production and almost all molecular O2 consumption occur at the level of oxidative phosphorylation in the mitochondria. The electrons carried by the reduced NADH and FADH2 generated in the citric acid cycle as well as by glycolysis and β-oxidation are transferred to the mitochondrial electron transport chain, which fuels phosphorylation of ADP to yield ATP. Molecular O2 is the terminal electron (and proton) acceptor in electron transport; O2 is reduced to produce H2O. In macronutrient oxidation, the release of CO2 generally occurs at earlier stages in nutrient catabolism and thus prior to O2 consumption and H2O production. The coupling of oxidation of fuel molecules to ATP synthesis (with the exception of some substrate-level ATP formation) is localized within the mitochondria of mammalian cells, as illustrated in Figure 21-3. The specialized proteins and enzymes required for oxidative phosphorylation are specifically localized on the inner mitochondrial membrane. Oxidative phosphorylation is the process by which a molecule of inorganic phosphate is condensed with ADP to form ATP, a process driven by the step-by-step transfer of electrons along a chain of electron carriers termed the electron transport chain (Figure 21-4). The synthesis of ATP during oxidative phosphorylation is linked to the release of electrons carried by the flavin and pyridine nucleotides to proteins of the electron transport chain. Hydrogen ions are pumped out of the mitochondrial matrix across the inner mitochondrial membrane at three sites along the electron transport chain to create an electrochemical gradient. This proton gradient is subsequently dissipated as protons are allowed back through the membrane by ATP synthase, which couples this process to ATP synthesis, as shown in Figure 21-4. Sufficient free energy may be collected at three sites along the electron transport chain (complexes I, III, and IV) to allow phosphorylation of ADP, yielding ATP. A maximum of three molecules of ATP can be generated from each molecule of NADH that is oxidized via donation of its pair of electrons to the electron transport chain. FADH2 donates its electrons at a later step in the electron transport chain, allowing a maximum of only two molecules of ATP to be generated from each FADH2 molecule. A frequently used index of the efficiency of oxidative phosphorylation is the P/O ratio. The P/O ratio is defined as the number of ATP molecules produced per pair of electrons traveling through the electron transport chain (or per oxygen atom that is reduced). Depending on where electrons enter the chain, the maximal P/O ratio will change (Murphy and Brand, 1987). At maximal biological efficiency, the complete oxidation of NADH yields 3 ATP molecules, and so the maximal P/O ratio for this process is 3. For the complete oxidation of FADH2, the maximal P/O ratio is 2. Few direct attempts to determine the P/O ratio in intact cells have been made because of the difficulties encountered in determining the rapid turnover of ATP molecules involved in the maintenance of a cell’s metabolic activities without disturbing the cell’s internal milieu. Nevertheless, the physiological P/O ratio is almost certainly somewhat less than the maximal values of 3 and 2 (probably closer to 2.5 and 1.5) that are commonly used as a basis for calculating the “ATP equivalents” produced by oxidation of NADH and FADH2, respectively, by the electron transport chain. Net ATP yield depends on the amount of energy (ATP equivalents) the cell must expend in the complete metabolism of the energy-yielding molecule and on that produced via substrate-level phosphorylation and via oxidative phosphorylation. For example, the complete oxidation of 1 molecule of glucose via glycolysis, conversion of pyruvate to acetyl-CoA, and oxidation of acetyl-CoA in the citric acid cycle (as described in Chapter 12) produces 34 ATPs by oxidative phosphorylation and 6 ATPs (or equivalent GTP) by substrate-level phosphorylation. However, 2 ATPs are consumed in glycolysis, so the net yield is 38 ATPs.
Cellular and Whole-Animal Energetics
Cellular Energetics
Adenosine Triphosphate and Nicotinamide Adenine Dinucleotide Phosphate
Metabolic Sources of Heat Production
Obligatory Heat Production
Regulatory Heat Production
Oxidation of Fuel Molecules
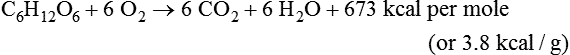
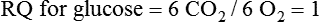
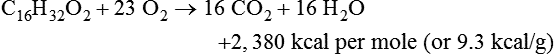
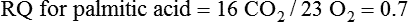
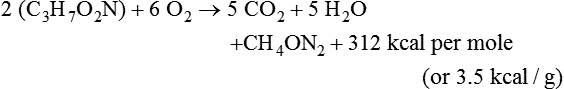
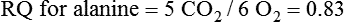
Oxidative Phosphorylation
Phosphorylation of ADP to ATP
P/O Ratio
Net ATP Production from Fuel Oxidation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine