Cardiac Function
Jacquelyn L. Banasik
Key Questions
• What factors affect the blood supply to myocardial tissue?
• How does sarcomere cross-bridge formation lead to muscle cell contraction?
• What is the process of excitation-contraction coupling in heart muscle cells?
• How are action potentials generated and conducted in myocardial and pacemaker cells?
• How does the electrocardiogram relate to impulse conduction through the heart?
• How do heart rate, preload, afterload, and contractility affect cardiac output and cardiac workload?
• What diagnostic tests are used to evaluate cardiac structure and function?
![]()
http://evolve.elsevier.com/Copstead/
The primary function of the heart is to produce the driving force that propels blood through the vessels of the circulatory system. Along with the lungs, the heart works to distribute oxygenated blood and nutrients to tissues and organs of the body. Complex regulatory mechanisms function to match the cardiac output with the metabolic needs of the tissues. Cardiac dysfunction can lead to abnormal function or death of cells in tissues throughout the body. Cardiovascular disease is the leading cause of mortality in the United States, and a significant proportion of the population suffers from physical limitations associated with impaired cardiac function. Familiarity with cardiac anatomy and physiology is requisite to understanding cardiac diseases and therapy.
Cardiovascular Anatomy
Heart
The heart is located in the mediastinum, suspended between the lungs, behind the sternum, and in front of the vertebral column, thoracic aorta, and esophagus (Figure 17-1).1 When viewed from the front, the heart appears to be rotated to the left, so that the right atrium and right ventricle are most anterior. The base of the heart protrudes somewhat into the right side of the chest and is relatively fixed in place by its attachments to the great vessels. The apex of the heart lies primarily in the left side of the chest and is directed forward toward the anterior chest wall. With each heartbeat, a characteristic thrust, or point of maximal impulse (PMI), is generated and can be palpated where the apex strikes against the chest. The PMI is normally located on the left side of the chest where the fifth intercostal space and midclavicular line intersect. Variations in heart size and position within the chest may be related to age, body size, shape, weight, or pathologic conditions of the heart and other nearby structures.
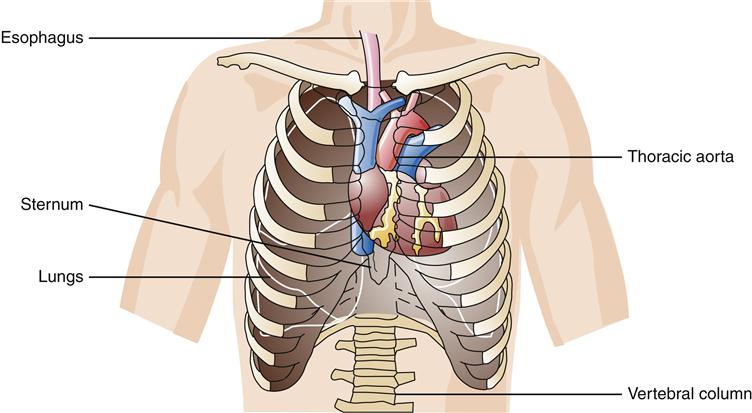
The base of the heart protrudes into the right side of the chest, whereas the apex lies in the lower left side of the chest.
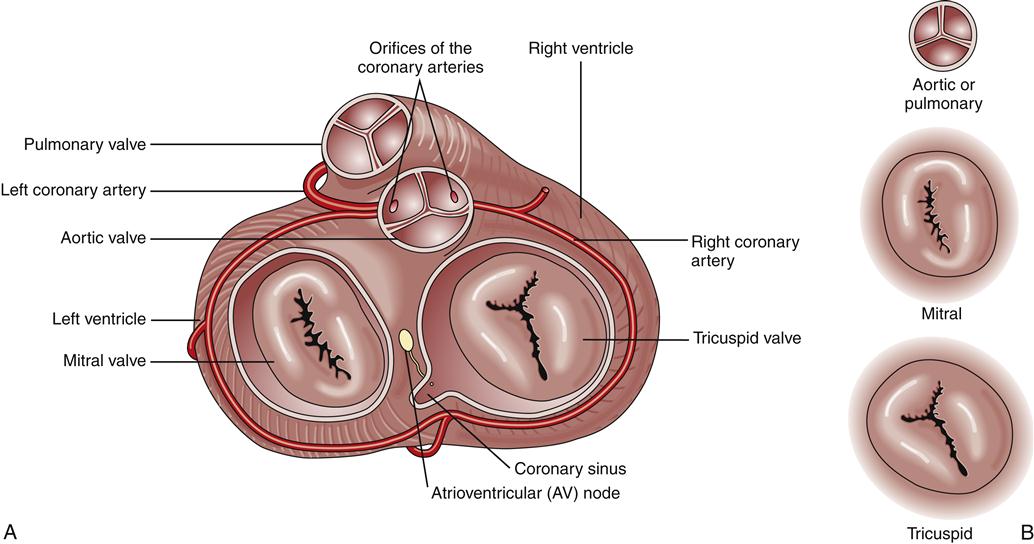
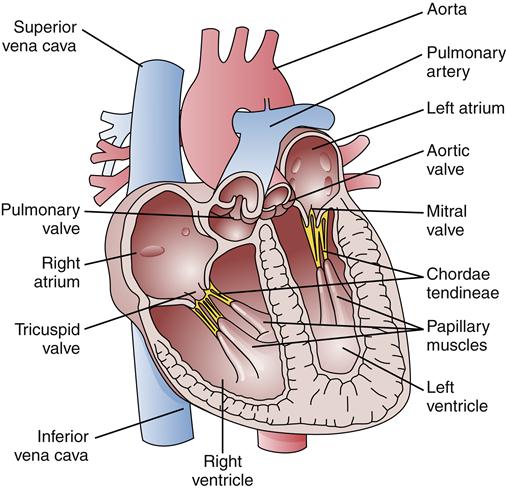
Functionally important cardiac tissues include connective tissues, which form the fibrous skeleton and valves; cardiac muscle, which produces the contractile force; and epithelial tissue, which lines the cardiac chambers and covers the outer surfaces of the heart. The fibrous skeleton includes an extensive network of matrix that supports cardiac cells and four rings that provide a firm scaffold for attachment of the cardiac valves. Four cardiac valves control the direction of blood flow through the heart (Figure 17-2). The mitral valve (bicuspid) directs blood flow from the left atrium to the left ventricle, whereas the tricuspid valve directs blood from the right atrium to the right ventricle. The edges of these atrioventricular (AV) valves are attached to rings formed by the fibrous skeleton. Valve leaflets are tethered to papillary muscles of the ventricular chambers by connective tissues called chordae tendineae. Papillary muscles attach to ventricular walls and help prevent the valve leaflets from bending backward into the atria during ventricular contraction (Figure 17-3). The AV valves open passively during diastole when the pressure of blood in the atria exceeds that in the ventricles. Ventricular contraction reverses the pressure gradient and causes AV valves to snap shut, preventing blood from flowing backward into the atria.
Two semilunar valves are located in the ventricular outflow tracts. The pulmonic valve lies between the right ventricle and pulmonary artery, and the aortic valve lies between the left ventricle and aorta. Compared to the AV valves, the semilunar valves are thicker and are not supported by fibrous cords. They open and close passively according to pressure gradients, just as the AV valves do. When intraventricular pressures exceed pulmonary and aortic pressures, the semilunar valves remain open and then close when ventricular pressures fall below aortic and pulmonary artery pressures.
The cardiac muscle layer (myocardium) produces the contractile force that pushes blood through the circulatory system. Heart muscle is organized into four separate chambers of varying muscular wall thickness, reflecting the degree of pressure each chamber must generate to pump blood. Atria serve primarily as conduits and have a thinner layer of muscle than the ventricles. The left ventricular muscle is two to three times thicker than that of the right ventricle because higher pressures are required to eject blood into the systemic circulation than into the pulmonic system. Normal chamber pressures are shown in Table 17-1. Alterations in chamber pressures may reflect pathologic cardiovascular changes such as valvular disorders, blood volume abnormalities, and heart failure (see Chapters 18 and 19).
TABLE 17-1
| LOCATION | PRESSURE (mm Hg)∗ |
| Right atrium | 0-8 |
| Right ventricle | 15-28/0-8 |
| Pulmonary artery | 15-28/4-12 |
| Left atrium | 4-12 |
| Left ventricle | 100-120/4-12 |
| Aorta | 100-120/60-80 |
∗Right and left atrial pressures listed as means; other pressures written as systolic/diastolic.
Cardiac chambers and valves are lined by a layer of squamous epithelial cells called the endocardium. The endocardial layer provides a smooth surface that prevents clotting and minimizes trauma to red blood cells. The endocardium is continuous with the endothelium of the vascular system. Outer surfaces of the heart are also covered by a layer of epithelial cells called the epicardium, which is part of a protective covering called the pericardium. The pericardium is composed of two layers that envelop the heart like a sac (Figure 17-4). The inner layer (visceral pericardium or epicardium) is attached directly to the heart’s outer surface, whereas an outer layer (parietal pericardium) forms a sac around the heart. The parietal pericardium is composed of an epithelial layer and a tough fibrous layer.
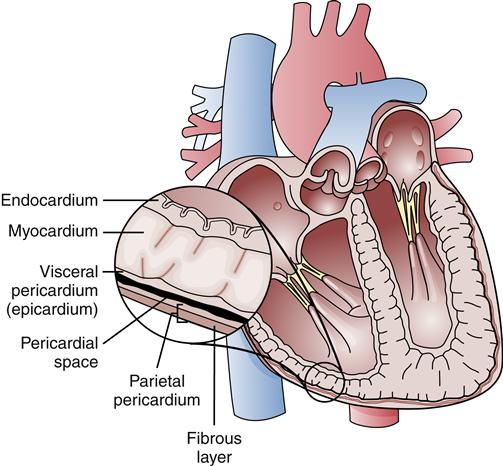
The visceral pericardium (epicardium) is attached directly to the heart’s surface, whereas the parietal pericardium forms the outer layer of the sac.
Visceral and parietal pericardial layers are separated by a thin, fluid-filled space (pericardial space) that usually contains 10 to 30 ml of serous fluid. This fluid lubricates pericardial surfaces and reduces friction while the layers slide against one another during cardiac contraction. Accumulations of fluid in the pericardial space or inflammation of the pericardial sac can restrict cardiac filling and impair cardiac output.
Circulatory System
The circulatory systems of the lungs and body can be viewed as two separate but interdependent systems (Figure 17-5). The left-sided heart chambers produce the force to propel blood through the vessels of the systemic (body) circulation. The left atrium receives oxygenated blood from the lungs by way of the pulmonary veins and delivers it to the left ventricle. This oxygenated blood is pumped by the left ventricle into the aorta, which supplies the arteries of the systemic circulation. Venous blood is collected from capillary networks of the body and returned to the right atrium by way of the vena cavae. Blood from the head returns to the right atrium through the superior vena cava; blood from the body returns via the inferior vena cava. There are no valves between the vena cavae and the right atrium, and the atrial pressure waves that are generated during the cardiac cycle cause characteristic visible pulsations in the jugular veins. An increased right atrial pressure may be observed as distention within the jugular veins.
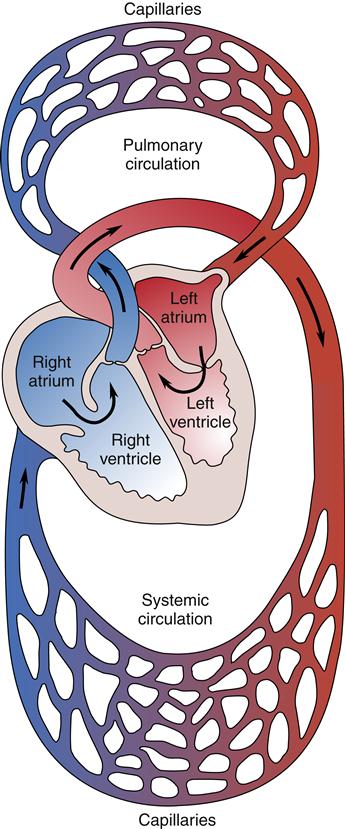
The right ventricle pumps blood through the pulmonary vasculature, whereas the left ventricle pumps blood through the systemic circulation.
The right side of the heart receives deoxygenated blood from the systemic circulation and pumps it through the lungs by way of the pulmonary artery. The pulmonary artery divides into left and right branches, which subdivide to supply blood to pulmonary capillary beds. Exchange of respiratory gases occurs at the pulmonary capillaries so that blood delivered to the left atrium by the pulmonary veins is well oxygenated.
Blood flow through the left and right heart chambers is connected in series such that the output of one becomes the input of the other. Thus, the functions of the right and left sides of the heart are interdependent. Failure of one side of the heart to pump efficiently soon leads to dysfunction of the other side.
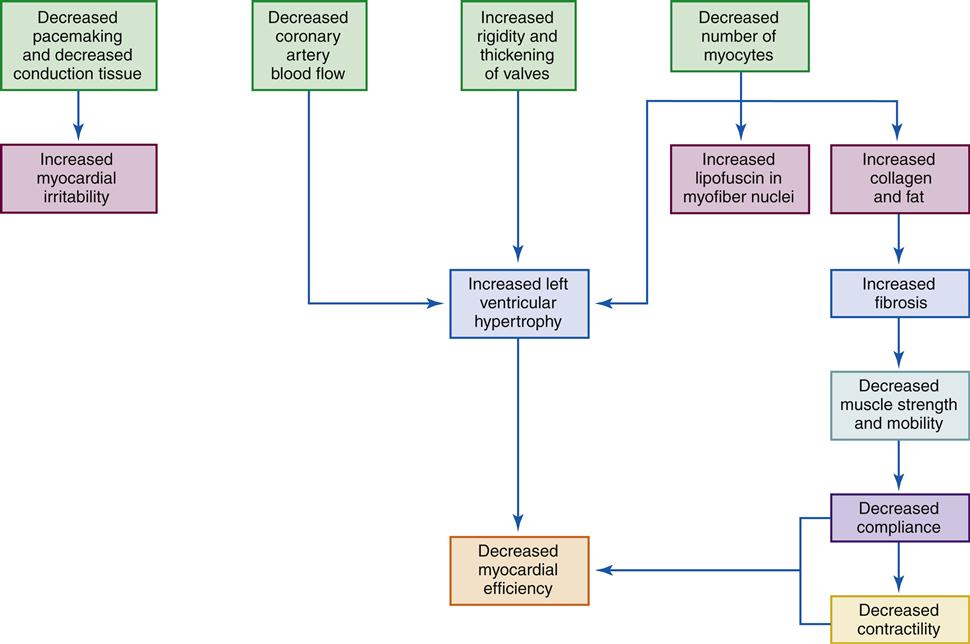
Characteristic changes in the anatomy and physiologic functioning of the heart and circulatory systems occur with aging (see Geriatric Considerations: Changes in the Heart). In general, these changes result in a decreased cardiac reserve and a greater predisposition to cardiac muscle ischemia.
Cardiac Cycle
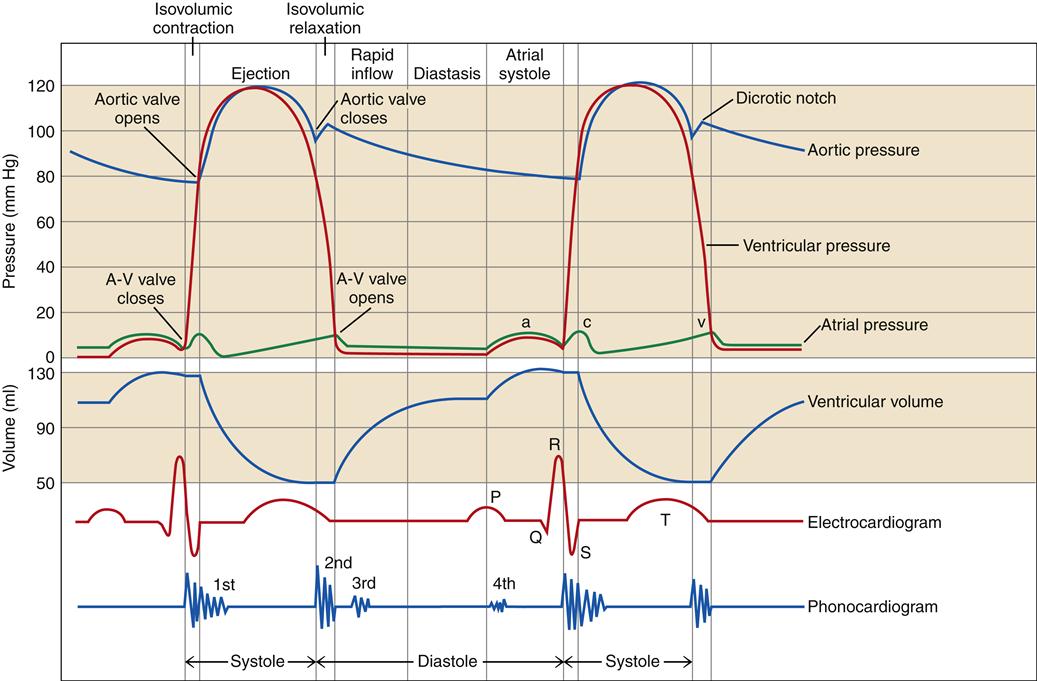
Each heartbeat is composed of a period of ventricular contraction (systole) followed by a period of relaxation (diastole). The interval from one heartbeat to the next is called a cardiac cycle and includes ventricular, atrial, and aortic (or pulmonic) events. Each of these events is associated with characteristic pressure changes within the cardiac chambers.2 Pressure changes result in valvular opening and closing and unidirectional movement of blood through the heart. The various events of the cardiac cycle are illustrated as a function of time in Figure 17-6. Another method of graphing ventricular function is the pressure-volume loop (Figure 17-7). Pressure-volume loops are useful for assessing the relationships between pressure and volume at various points in the cardiac cycle to evaluate left ventricular function. Abnormalities in these waveforms may occur with diseases of the cardiac valves, changes in blood volume, or changes in pumping capacity of the heart (see Chapter 18). These waveforms are commonly monitored with specialized cardiac catheters in patients with cardiac or hemodynamic disorders.
An identical set of events occurs on the right side of the heart, although pressures are lower.
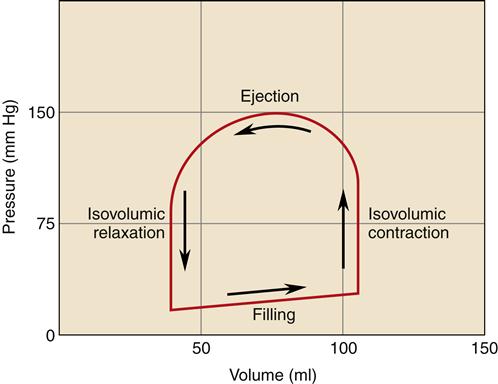
The cardiac cycle can be described sequentially, beginning with ventricular filling. During diastole the ventricles are relaxed and blood flows in from the atria through open AV valves. Initially, ventricular filling occurs passively because of a pressure gradient between the atria and ventricles. Toward the end of ventricular diastole, the atria contract, squeezing more blood through the AV valves into the ventricles. The “atrial kick” provided by atrial contraction is particularly important during fast heart rates, when the time for ventricular filling is shortened; the atrial contraction helps to load the ventricle quickly to prevent a reduction in stroke volume. Ventricular events include isovolumic contraction, ejection, and isovolumic relaxation. Each of these cycle events is further described in the following sections.
Isovolumic Contraction
Immediately following atrial systole the ventricles begin to contract, causing intraventricular pressure to rise and the AV valves to close. AV valve closure produces a sound that can be heard at the chest wall and is termed S1. Ventricular pressure rises rapidly during isovolumic contraction because all four cardiac valves are closed, and the volume of blood within the ventricular chamber is forcefully compressed by the powerful ventricular myocardium (see Figure 17-6, red tracing). Volume remains constant during this phase. The rate of rise in pressure is an indication of the contractile state of the heart. The greater the change in pressure per unit time (dP/dt), the higher the contractile state. Sympathetic nervous system activation increases dP/dt whereas conditions such as heart failure are characterized by a slower rate of pressure development. The term inotropy is commonly used interchangeably with contractility and is reflected by the velocity and degree of cardiac muscle shortening during systole.
Ventricular Ejection
Ventricular contraction results in a rapid rise in ventricular pressure. As ventricular pressure exceeds aortic pressure (or pulmonic), the valve is forced open and a period of rapid ejection of blood from the ventricle follows. The rapid ejection phase is followed by a period of reduced ejection as aortic (or pulmonic) pressure rises and ventricular pressures and volumes fall. The amount of blood ejected with each contraction of the ventricle is called the stroke volume (SV). The volume of blood in the ventricle before ejection is the end-diastolic volume (EDV) and the amount of blood that remains in the ventricle after ejection is the end-systolic volume (ESV). Thus, stroke volume equals EDV minus ESV. An important and commonly used index of pumping effectiveness is the ejection fraction (EF), which is calculated by dividing SV by EDV. A normal EF is
60% to 80%; patients with systolic heart failure often have an EF of less than 40%.
Isovolumic Relaxation
The isovolumic relaxation phase begins with semilunar valve closure in response to falling ventricular pressures and ends when the AV valves open to allow ventricular filling. Ventricular blood volume remains constant during this period because all four cardiac valves remain closed. Closure of the semilunar valves causes the second heart sound, S2. Opening of the AV valves signals the beginning of rapid ventricular filling and the start of another cardiac cycle. The rate of ventricular relaxation is indicated by the drop in ventricular pressure per unit time and is called the −dP/dt. The rate and degree of ventricular relaxation is called lusitropy and is an energy-requiring process that reflects the efficiency of calcium removal from the cytoplasm. Rapid relaxation is necessary to allow the ventricle to fill quickly and at a low pressure before the next systole. Impaired relaxation (lusitropic dysfunction) is a common finding in patients with heart failure and contributes to the symptoms of congestion (see Chapter 19). Because relaxation of the ventricle is an energy-requiring process, it may become impaired when blood flow and oxygen delivery to the heart are inadequate.
Atrial Events
Atrial pressure waves have three characteristic curves: a, c, and v (see Figure 17-6, green tracing). The a wave corresponds to atrial contraction, which immediately precedes AV valve closure. The c wave occurs early in ventricular systole and is thought to represent bulging of AV valves into the atrial chambers. The v waves have a gradual incline, which represents filling of the atrium as blood returns from the circulation. The v wave drops rapidly as atrial pressure is relieved by AV valve opening. A large v wave is often associated with inadequate closure of the AV valve, resulting in regurgitation of ventricular blood back into the atrium during ventricular systole. The mean right atrial pressure, also called the central venous pressure (CVP), is commonly measured as an indicator of the blood volume in the heart, which is dependent in part on the amount of blood being returned from the systemic circulation.
Aortic and Pulmonary Artery Events
Aortic and pulmonary artery pressures rise and fall in relation to the cardiac cycle. Arterial pressures fall to their lowest value just before semilunar valve opening. This lowest pressure is called diastolic blood pressure. Arterial pressure reaches its maximum during ventricular ejection and is called systolic blood pressure. A characteristic notch (dicrotic notch) in the arterial pressure curve may be seen as the semilunar valves close (see Figure 17-6, blue tracing).
The difference in aortic pressure between systole and diastole is partly dependent on the aorta’s elastic characteristics. During systole, the aorta stretches to accommodate blood ejected by the ventricle. The stretched aorta has “stored” or potential energy that is released during diastole to maintain driving pressure and to keep blood flowing continuously through the circulation. Aortic stiffening, as occurs with aging or arteriosclerosis, may result in higher systolic and lower diastolic blood pressures attributable to loss of aortic elastic properties.3 When aortic or pulmonic pressures are chronically elevated, the ventricles must generate more pressure to open the semilunar valves and eject the stroke volume. Over time this extra effort required to increase the pressure can damage the heart muscle and lead to hypertrophy or failure.
Coronary Circulation
Anatomy of the Coronary Vessels
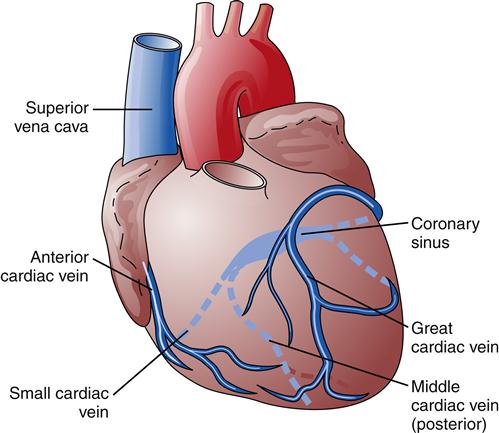
The blood supply to heart muscle is provided by the coronary arteries (Figure 17-8). Right and left coronary artery openings are located in the sinuses of Valsalva, in the aortic root, just beyond the aortic valve.2 The right coronary artery originates near the aortic valve’s anterior cusp and passes diagonally toward the right ventricle in the AV groove. In approximately 50% of the population, the right coronary artery gives rise to a posterior descending vessel that supplies blood to the heart’s posterior aspect. In 20% of the population, the left coronary artery is dominant in supplying blood to the ventricles, and in 30% of the population the right and left coronary arteries deliver about the same amount of blood and neither is dominant.3 The left main coronary artery arises near the aortic posterior cusp and travels a short distance anteriorly before dividing into the left anterior descending and circumflex branches. The anterior descending branch supplies septal, anterior, and apical areas of the left ventricle, whereas the circumflex artery supplies the lateral and posterior left ventricle. The three major coronary arteries give rise to a number of smaller branches that penetrate the myocardium and branch into small arterioles and capillaries. Regular exercise and stable atherosclerotic plaques in the coronary arteries are thought to stimulate the development of more extensive collateral circulation in the heart. Collateral vessels may help limit infarct size in patients suffering acute coronary occlusions (see Chapter 18). Areas supplied by divisions of the coronary arteries are listed in Table 17-2. Most of the heart’s capillary beds drain into the coronary veins, which then empty into the right atrium through the coronary sinus (Figure 17-9).
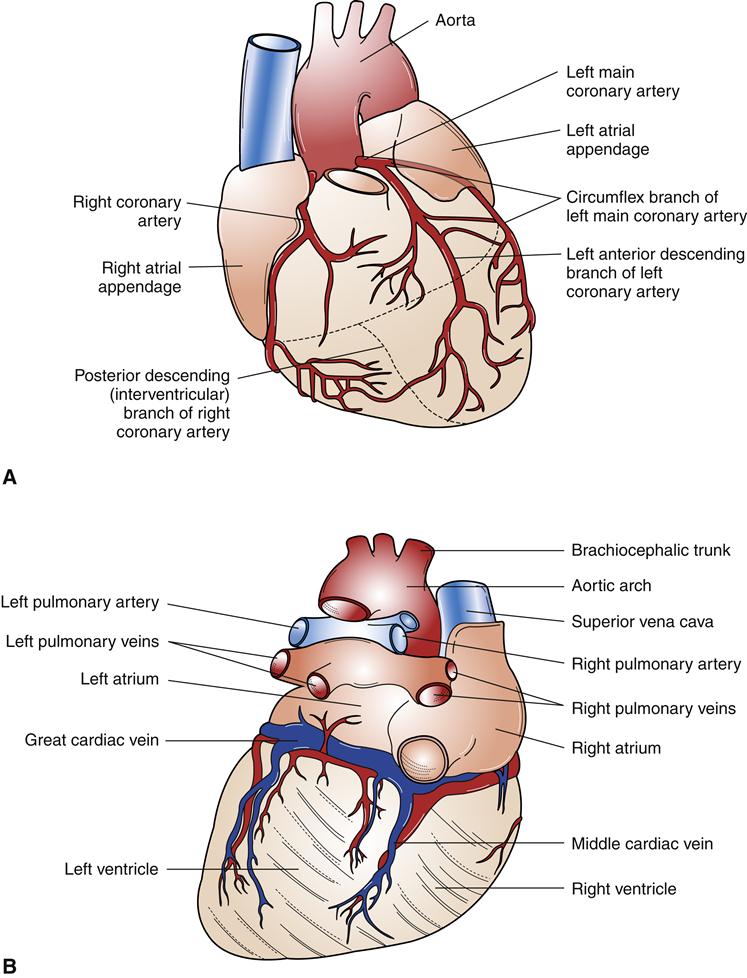
The right coronary artery supplies the right atrium, ventricle, and posterior aspect of the left ventricle in most individuals. The left coronary artery divides into the left anterior descending and circumflex arteries, which perfuse the left ventricle. A, Anterior view. B, Posterior view.
TABLE 17-2
AREAS SUPPLIED BY THE CORONARY ARTERIES
| ARTERY | AREA SUPPLIED |
| Right coronary | Right atrium (55% of persons) Right ventricle Intraventricular septum Sinus node (55% of persons) Atrioventricular node Bundle of His |
| Left anterior descending | Right atrium (45% of persons) Right ventricle (minor) Left ventricle (anterior, apex) Anterior papillary muscles Right and left bundle branches Intraventricular septum |
| Left circumflex | Left atrium Left ventricle (posterior, anterior) Sinus node (45% of persons) |
Regulation of Coronary Blood Flow
Blood flow through coronary vessels is determined by the same physical principles that govern flow through other vessels of the body, namely, driving pressure and vascular resistance to flow.3 According to Ohm’s law, an increase in driving pressure (P) increases blood flow (Q), whereas an increase in resistance (R) reduces blood flow: Q = P/R (see Chapter 15). Driving pressure through the coronary arteries is determined by aortic blood pressure and right atrial pressure. This relationship can be expressed in the following equation:
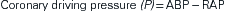
where ABP is aortic blood pressure and RAP is right atrial pressure. Thus, an increase in aortic pressure enhances coronary blood flow, whereas an increase in right atrial pressure opposes coronary flow.
Coronary vascular resistance (R) has two major determinants: (1) coronary artery diameter and (2) the varying degrees of external compression attributable to myocardial contraction and relaxation. Coronary artery diameter is continuously adjusted to maintain blood flow at a level adequate for myocardial demands. Autoregulation is the term used to describe the intrinsic ability of the arteries to adjust blood flow according to tissue needs. Vessel dilation (vasodilation) occurs in response to increased tissue metabolism or reduced driving pressure, whereas decreased metabolic activity or increased driving pressure results in a decreased vessel diameter (vasoconstriction).
The mechanism of autoregulation can be explained by the metabolic hypothesis, which proposes that increased metabolism, reduced oxygen concentration, or decreased blood flow results in a buildup of vasodilatory chemicals in the vessel. Smooth muscle encircling the vessel relaxes in response to the presence of the chemicals, increasing vessel diameter. Several vasodilating substances have been proposed, including potassium ions, hydrogen ions, carbon dioxide, nitric oxide, prostaglandins, and adenosine. The endothelial cells that line vessels are known to secrete a variety of relaxing and constricting factors, which may contribute to autoregulation.4 Vasodilatory substances are washed away as blood flow increases in response to increased vessel diameter. A declining level of vasodilatory chemicals results in vasoconstriction. Thus, vessel diameter is continuously adjusted according to concentrations of vasodilatory chemicals, which are directly related to the tissue’s metabolic activity.
One mechanism for autoregulation of coronary blood flow involves an ATP-sensitive potassium channel in vascular smooth muscle.5 When ATP levels rise in response to increased coronary flow, the channel closes, making it easier to depolarize the cell and contract vascular smooth muscle. Contraction of vascular smooth muscle reduces the diameter of the coronary arteries and reduces blood flow. The opposite also occurs: a reduction in ATP level, due to low flow or increased metabolism, opens the K+ channels. Potassium then leaks out of the vascular smooth muscle and short-circuits the depolarizing influences. This inhibits vascular contraction, leading to vasodilation and increased coronary blood flow. Adenosine also contributes to regulation of the ATP-sensitive K+ channels, causing vasodilation when adenosine levels are elevated.
Nitric oxide (NO) produced by endothelial cells lining the coronary arteries is an important regulator of coronary blood flow. NO is a diffusible gas produced by the enzyme inducible nitric oxide synthase in response to numerous stimuli including hypoxemia and platelet factors. NO is a potent vasodilator, and inhibition of its production is associated with reduced coronary blood flow. Many known risk factors for coronary heart disease have been shown to impair nitric oxide–dependent vasodilation of coronary arteries.5
Vessel diameter also is regulated by the autonomic nervous system. The coronary arteries are primarily innervated by sympathetic nerves, but they also receive a small amount of parasympathetic innervation. The sympathetic neurotransmitter norepinephrine (NE) binds to both α1 and β2 receptors in coronary arteries; α1 stimulation results in vasoconstriction, whereas β2 stimulation dilates. Under normal conditions the vasodilator response predominates, but in pathologic states, excessive α1-mediated constriction can occur. The increased metabolic activity associated with sympathetic nervous system stimulation generally causes autoregulatory vasodilation and overrides the direct effect of norepinephrine on the vessels. Parasympathetic activity contributes to vasodilation by promoting the production of nitric oxide by coronary endothelial cells.
In addition to vessel diameter, coronary resistance is affected by myocardial contraction. During systole, cardiac muscle compression creates a marked rise in coronary resistance that reduces coronary blood flow (perfusion). Blood flow to the left ventricle is greatly decreased during systole because of the pressures generated by the thick muscular layer. Blood vessels that penetrate the myocardium to supply the innermost endocardial areas are more compressed during contraction than are outer epicardial vessels. Even though coronary artery driving pressure is greatest during ventricular systole, little blood flow reaches the left ventricle because of the high external pressure applied to the coronary vessels as the myocardium contracts. Therefore, most myocardial blood flow occurs during the diastolic interval between ventricular contractions. The time the heart spends in diastole is directly related to heart rate. Faster heart rates reduce diastolic time and decrease coronary artery blood flow.
Cardiac muscle needs a continuous supply of oxygen and nutrients to perform its pumping functions. A disruption in cardiac blood flow (ischemia) generally results in some degree of pump failure and damage to cardiac tissues. Myocardial ischemia may be caused by conditions that reduce coronary blood flow or increase myocardial demands for oxygen. These include (1) reduced driving pressure (e.g., low aortic blood pressure or high right atrial pressure), (2) reduced vessel diameter (e.g., myocardial hypertrophy, arteriosclerosis, thrombosis, vasoconstricting chemicals), (3) reduced perfusion time (e.g., high heart rates, some dysrhythmias), and (4) increased metabolic demands (e.g., fever, sepsis, anemia).
Cardiac Myocytes
Cardiac muscle cells are divided into two general types: working cells, which have primarily mechanical pumping functions, and electrical cells, which primarily transmit electrical impulses. Both types are excitable: they are able to produce and transmit action potentials. Working myocardial cells are packed with contractile filaments and compose the bulk of the atrial and ventricular muscle. Electrical cells function to initiate and coordinate contraction of the working cells. Differentiated cardiac myocytes are unable to enter the cell cycle to proliferate; however, they can increase in size and synthesize more contractile proteins (hypertrophy). New myocardial cells can be formed from stem cells that have the potential to divide. Stem cells may be recruited from the circulation and stimulated to divide and mature into myocytes within the myocardium. Conditions that increase myocardial cell death are thought to stimulate recruitment of stem cells into the myocardium. A high turnover rate of cardiac myocytes occurs, and increases with age, suggesting that the entire population of cells within the heart is completely replaced 11 to 15 times over a lifetime.6 When the rate of myocardial cell loss exceeds replacement by stem cells, the condition of heart failure may ensue (see Chapter 19).
Myocyte Structure
Typical myocardial cells (myocytes) are illustrated in Figure 17-10. Cardiac myocytes are described as muscle “fibers” because of their long, narrow shape. The plasma membrane (sarcolemma) of one cardiac cell is joined end-to-end with its neighbors by intercalated disks, which contain gap junctions that allow the rapid passage of electrical impulses from one cell to the next. The intercalated disks permit the many separate cells of the myocardium to function together in a coordinated manner. This arrangement is called a functional syncytium. The sarcolemma also forms membrane-lined channels that penetrate the cell and become the transverse tubules (T tubules) (Figure 17-11). The T tubules permit extracellular fluid and ions to diffuse near intracellular structures. Movement of ions across the sarcolemma is an essential part of cellular excitation and the subsequent contraction of intracellular elements. Cellular contractile elements are simultaneously activated because signals at the cell surface are rapidly transmitted internally by the T tubules.7
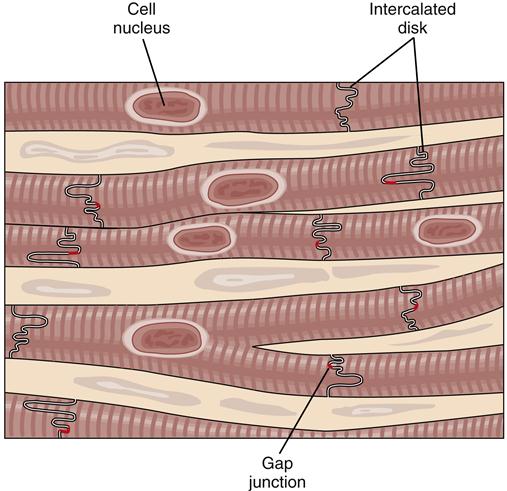
The end of one muscle cell is fused to the next by intercalated disks. Within these connections are specialized proteins that form a fluid-filled pore (gap junction) between the fused cells. Ions can travel through the gap junctions to transport changes in membrane potential from one cell to the next.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree