The antiseizure activity of carbamazepine is related to its ability to decrease transmission in the nucleus ventralis anterior section of the thalamus, an area of the brain thought to be involved with the generalization and propagation of epileptic discharges.7,10 Although the exact cellular mechanism of action is unclear, inhibition of voltage-gated sodium channels appears to be involved. Additionally, carbamazepine depresses post-tetanic potentiation and may prevent increases in cyclic adenosine monophosphate (cAMP).
THERAPEUTIC AND TOXIC CONCENTRATIONS
The accepted therapeutic range for carbamazepine is 4-12 μg/mL when the drug is used for the treatment of seizures. Carbamazepine plasma protein binding is quite variable among individuals because it is bound to both albumin and α1-acid glycoprotein (AGP). In patients with normal concentrations of these proteins, plasma protein binding is 75%-80% resulting in a free fraction of drug of 20%-25%.11–14 AGP is classified as an acute-phase reactant protein that is present in lower amounts in all individuals but is secreted in large amounts in response to certain stresses and disease states such as trauma, heart failure, and myocardial infarction. In patients with these disease states, carbamazepine binding to AGP can be even larger resulting in an unbound fraction as low as 10%-15%.
Little prospective work has been done to establish the therapeutic range for unbound carbamazepine serum concentrations or the clinical situations where unbound carbamazepine serum concentration measurement is useful. As an initial guide, 25% of the total carbamazepine therapeutic range as been used to establish a preliminary desirable range for unbound carbamazepine serum concentrations of 1-3 μg/mL. Although carbamazepine is highly plasma protein bound, it is harder to displace this agent to the extent that a clinically important change in protein binding takes place. Generally speaking, a doubling in unbound fraction in the plasma is required to produce such an alteration. In comparison, phenytoin is 90% protein bound under usual circumstances resulting in an unbound fraction in the plasma of 10%. It is relatively easy to change the protein binding of phenytoin from 90% to 80%, under a variety of disease states or conditions, which increases the unbound fraction in the plasma from 10% to 20%. However, it is very difficult to change the protein binding of carbamazepine from 80% to 60% to achieve the same doubling of unbound fraction in the plasma (20%-40%). As a result of this, the use of unbound carbamazepine serum concentrations are currently limited to those patients that have total concentrations within the therapeutic range but experience adverse effects usually seen at higher concentrations, or those patients that have total concentrations below the therapeutic range but have a therapeutic response usually observed at higher concentrations.
Carbamazepine-10, 11-epoxide is an active metabolite of carbamazepine that contributes to both the therapeutic and toxic effects of the drug, and can be measured in serum samples at a limited number of epilepsy centers.15–21 The concentration of the epoxide is often related to the presence or absence of other inhibitors or inducers of hepatic drug-metabolizing enzymes. Epoxide concentrations tend to be higher in patients taking enzyme inducers and lower in patients taking enzyme inhibitors. The percent of epoxide to parent drug in chronically treated patients averages about 12% for carbamazepine monotherapy, 14% when carbamazepine is taken with phenobarbital, 18% when carbamazepine is taken with phenytoin, and about 25% when carbamazepine is taken with both phenytoin and phenobarbital. Currently, the therapeutic range of carbamazepine-10, 11-epoxide is not known although a suggested range of 0.4-4 μg/mL is used by several research centers.
In the upper end of the therapeutic range (>8 μg/mL) some patients will begin to experience the concentration-related adverse effects of carbamazepine treatment: nausea, vomiting, lethargy, dizziness, drowsiness, headache, blurred vision, diplopia, unsteadiness, ataxia, incoordination. Because carbamazepine induces its own hepatic metabolism, these adverse effects can also be seen early during dosage titration periods soon after dosage increases are made. To improve patient acceptance, it is important to initiate and titrate carbamazepine doses at a slow rate to minimize side effects. Clinicians should understand that all patients with “toxic” carbamazepine serum concentrations in the listed ranges will not exhibit signs or symptoms of carbamazepine toxicity. Rather, carbamazepine concentrations in the ranges given increase the likelihood that an adverse drug effect will occur.
CLINICAL MONITORING PARAMETERS
The goal of therapy with anticonvulsants is to reduce seizure frequency and maximize quality of life with a minimum of adverse drug effects. While it is desirable to entirely abolish all seizure episodes, it may not be possible to accomplish this in many patients. Patients should be monitored for concentration-related side effects (nausea, vomiting, lethargy, dizziness, drowsiness, headache, blurred vision, diplopia, unsteadiness, ataxia, incoordination). Because carbamazepine has antidiuretic effects associated with reduced levels of antidiuretic hormone, some patients may develop hyponatremia during chronic therapy with carbamazepine, and serum sodium concentrations can be periodically measured.
Hematologic adverse effects can be divided into two types. The first is a leukopenia that occurs in many patients and requires no therapeutic intervention. The typical clinical picture is an individual with a normal white blood cell count who develops a transient decrease in this index. In a few patients, a decreased, stable white blood cell count of 3000 cells/mm2 or less may persist and does not appear to cause any deleterious effects. The second hematologic effect is severe and usually requires discontinuation of the drug. Thrombocytopenia, leukopenia (trend downward in white blood cell count with <2500 cells/mm2 or absolute neutrophil count <1000 cells/mm2), or anemia are in this category. Rarely, aplastic anemia and agranulocytosis has been reported during carbamazepine treatment. Drug-induced hepatitis due to carbamazepine therapy has also been reported. The severe hematologic and hepatic adverse effects tend to occur early in treatment. Because of this, many clinicians measure a complete blood cell count and liver function tests monthly for the first 3-6 months after a patient first begins carbamazepine treatment, and repeat these tests every 3-6 months for the first year. Other idiosyncratic side effects include skin rash, Stevens-Johnson syndrome, and systemic lupus-like reactions.
Carbamazepine serum concentrations should be measured in most patients. Because epilepsy is an episodic disease state, patients do not experience seizures on a continuous basis. Thus, during dosage titration it is difficult to tell if the patient is responding to drug therapy or simply is not experiencing any abnormal central nervous system discharges at that time. Carbamazepine serum concentrations are also valuable tools to avoid adverse drug effects. Patients are more likely to accept drug therapy if adverse reactions are held to the absolute minimum. Because carbamazepine induces its own hepatic metabolism, it is fairly easy to attain toxic concentrations with modest increases in drug dose before maximal enzyme induction has occurred.
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
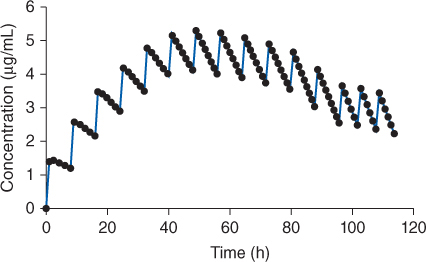
Carbamazepine is primarily eliminated by hepatic metabolism (>99%) mainly via the CYP3A4 enzyme system.22,23 Altogether 33 metabolites have been identified with carbamazepine-10, 11-epoxide being the major species. The epoxide metabolite is active and probably contributes to both the therapeutic and toxic side effects observed during therapy. Carbamazepine is a potent inducer of hepatic drug-metabolizing enzymes, and induces it’s own metabolism, a process known as autoinduction (Figures 11-1, 11-2).24–28 As a result, patients cannot initially be placed on the dose of carbamazepine that will ultimately result in a safe and effective outcome. At first, patients are started on ¼-⅓ of the desired maintenance dose. This exposes hepatic drug-metabolizing enzymes to carbamazepine and begins the induction process. The dose is increased by a similar amount every 2-3 weeks until the total desired daily dose is ultimately given. This gradual exposure of carbamazepine allows liver enzyme induction and carbamazepine clearance increases to occur over a 6-12 week time period. Therapeutic effect and steady-state carbamazepine serum concentrations can be assessed 2-3 weeks after the final dosage increase. Autoinduction continues to occur in patients who are stabilized on a carbamazepine dose but require a dosage increase. It appears that a 2-3 week time period is also needed under chronic dosing conditions for maximal autoinduction to occur after a dosage increase. The effects of autoinduction are reversible even when doses are held for as few as 6 days.29
FIGURE 11-1 Carbamazepine induces its own metabolism via the hepatic microsomal enzyme system CYP3A4 system. This process is known as autoinduction. When dosing is initiated, serum concentrations increase according to the baseline clearance and half-life. After a few doses of carbamazepine, enough autoinduction has occurred that clearance increases, half-life decreases, and drug accumulation slows down. With additional exposure of liver tissue to carbamazepine, clearance continues to increase and half-life continues to shorten. As a result of these pharmacokinetic changes, carbamazepine concentrations decline and ultimately stabilize in accord with the new clearance and half-life values. Maximal autoinduction usually occurs 2-3 weeks after dosing commenced. Because of the autoinduction phenomenon, the ultimate desired maintenance dose cannot be started with the first dose. Additional autoinduction occurs with subsequent increases in dose.
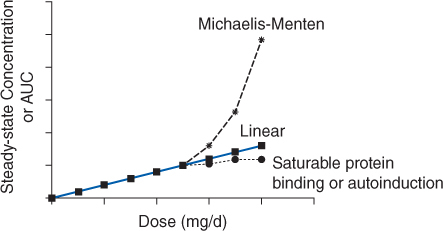
FIGURE 11-2 If a drug follows linear pharmacokinetics, Css or AUC increases proportionally with dose resulting in a straight line on the plot. Nonlinear pharmacokinetics occurs when the Css or AUC versus dose plot results in something other than a straight line. If a drug follows Michaelis-Menten pharmacokinetics (eg, phenytoin, aspirin), as steady-state drug concentrations approach Km serum concentrations increase more than expected due to dose increases. If a drug follows nonlinear protein binding (eg, valproic acid, disopyramide) or demonstrates autoinduction (eg, carbamazepine), total steady-state drug concentrations increase less than expected as dose increases.
An injectable form of carbamazepine is not available for clinical use. For oral use, the drug is available as immediate-release tablets (chewable: 100 mg, regular: 200 mg), extended-release tablets (100, 200, 400 mg), extended-release capsules (100, 200, 300 mg), and suspension (100 mg/5 mL). The rapid release dosage forms are erratically absorbed from the gastrointestinal tract resulting in peak concentrations between 2 and 24 hours after a single dose of tablets (average 6 hours). During multiple dose studies after maximal autoinduction has taken place, peak concentrations occur about 3 hours after tablet administration. Peak concentrations after multiple doses of the extended-release dosage forms are observed 3 to 12 hours after administration. Rectal administration of an extemporaneously compounded carbamazepine retention enema results in similar serum concentrations as that produced by a comparable immediate-release tablet.30,31
The absolute oral bioavailability of commercially available carbamazepine dosage forms for adult epileptics determined using an experimental intravenous injection for comparison is 78%.32 The relative oral bioavailability of other dosage forms (chewable tablet, suspension, extended-release tablets, and extended-release capsules) compared to the immediate-release tablet approaches 100%. If a patient is receiving a stable dose of carbamazepine on one dosage form, the same total daily dose of another dosage form can typically be substituted without adjustment. However, some bioequivalence problems have been reported for generic carbamazepine products.33–35
Usual initial maintenance doses are 10-20 mg/kg/d for children under 6 years of age, 200 mg/d for children 6-12 years old, and 400 mg/d for adults. Twice daily dosing is initially used until autoinduction takes place. Dosage increases to allow for autoinduction are made every 2-3 weeks depending on response and adverse effects. Most adults will require 800-1200 mg/d of carbamazepine while older children will require 400-800 mg/d. Although some minor side effects occur, single loading doses of 8 mg/kg have been given to adults as suspension or immediate-release tablets in order to achieve therapeutic concentrations within 2-4 hours after administration.36
EFFECTS OF DISEASE STATES AND CONDITIONS ON PHARMACOKINETICS AND DOSING
After single doses of carbamazepine, the oral clearance (Cl/F) is 11-26 mL/h/kg and half-life is 35 hours for adults.37–39 During multiple dosing after maximal autoinduction has taken place, oral clearance equals 50-100 mg/h/kg and half-life equals 5-27 hours. In children 6-12 years old, oral clearance and half-life equal 50-200 mL/h/kg and 3-15 hours, respectively, during chronic dosing. Clearance rates can be higher and half-lives shorter in patients receiving other hepatic drug-metabolizing enzyme inducers (phenytoin, phenobarbital, rifampin).40–42 Carbamazepine volume of distribution using immediate-release tablets (V/F) is 1-2 L/kg. The volume of distribution (V) using an experimental intravenous form of the drug is 1.1 L/kg.32
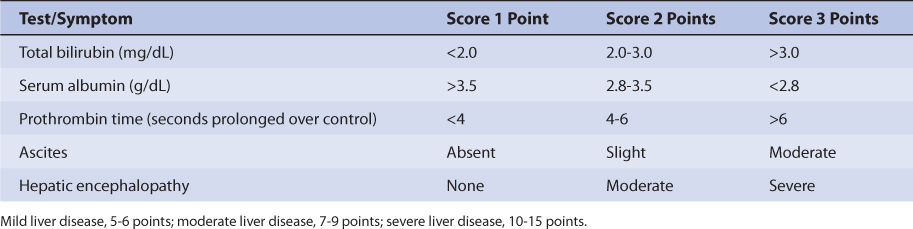
Patients with liver cirrhosis or acute hepatitis have reduced carbamazepine clearance because of destruction of liver parenchyma. This loss of functional hepatic cells reduces the amount of CYP3A4 available to metabolize the drug and decreases clearance. The volume of distribution may be larger because of reduced plasma protein binding. Protein binding may be reduced and unbound fraction maybe increased due to hypoalbuminemia and/or hyperbilirubinemia (especially albumin ≤3 g/dL and/or total bilirubin ≥2 mg/dL). However, the effects that liver disease has on carbamazepine pharmacokinetics are highly variable and difficult to accurately predict. It is possible for a patient with liver disease to have relatively normal or grossly abnormal carbamazepine clearance and volume of distribution. For example, a liver disease patient who has relatively normal albumin and bilirubin concentrations can have a normal volume of distribution for carbamazepine. An index of liver dysfunction can be gained by applying the Child-Pugh clinical classification system to the patient (Table 11-2).43 Child-Pugh scores are completely discussed in Chapter 3 (Drug Dosing in Special Populations: Renal and Hepatic Disease, Dialysis, Heart Failure, Obesity, and Drug Interactions), but will be briefly discussed here. The Child-Pugh score consists of five laboratory tests or clinical symptoms: serum albumin, total bilirubin, prothrombin time, ascites, and hepatic encephalopathy. Each of these areas is given a score of 1 (normal) to 3 (severely abnormal; see Table 11-2), and the scores for the five areas are summed. The Child-Pugh score for a patient with normal liver function is 5 while the score for a patient with grossly abnormal serum albumin, total bilirubin, and prothrombin time values in addition to severe ascites and hepatic encephalopathy is 15. A Child-Pugh score greater than 8 is grounds for a decrease of 25%-50% in the initial daily drug dose for carbamazepine. As in any patient with or without liver dysfunction, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Carbamazepine serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with liver cirrhosis.
Elderly and younger epileptic patients have similar carbamazepine clearances.14,32 However, older patients may be more sensitive to the central nervous system adverse effects of carbamazepine, and a lower starting dose of 100 mg/d is often used in elderly patients to avoid these problems. Compared to men, women have ∼26% higher clearance rates for carbamazepine.32 Carbamazepine clearance is ∼19% lower in African American patients compared to Caucasian patients.14,32 During the third trimester of pregnancy, oral clearance of carbamazepine may decrease and require dosage adjustment. Doses of carbamazepine do not require adjustment for patients with renal failure, and the drug is not removed by dialysis.44,45 Breast milk concentrations of carbamazepine are about 60% of concurrent serum concentrations.
DRUG INTERACTIONS
Carbamazepine is a potent inducer of hepatic drug-metabolizing enzyme systems and P-glycoprotein.46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree