Cancer Cachexia1
Vickie E. Baracos
1Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; EPCRC, European Palliative Care Research Collaborative.
The presence of a malignant disease is often heralded by a lowered intake of nutrients; however, important distinctions can be made between simple malnutrition and the tumor-bearing state. The pathophysiology of cancer cachexia is characterized by negative protein and energy balance, driven by a variable combination of reduced food intake and concurrent metabolic abnormalities. In addition to the presence of primary anorexia, a legion of nutrition impact symptoms poses barriers to food intake. Superimposed hypermetabolism and hypercatabolism accelerate depletion of physiologic reserves of energy and protein. There is merit in recognizing the onset of cachexia so that nutritional and metabolic interventions to reduce or delay its impact may be implemented. At later stages, cachexia may be clinically refractory because of the presence of rapidly progressive cancer unresponsive to antineoplastic therapy, and the focus of nutrition support shifts away from physiologic and functional outcomes to improvement of food enjoyment and quality of life.
CACHEXIA IS DISEASE-ASSOCIATED MALNUTRITION
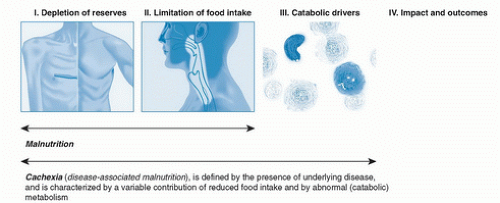
Multiple definitions and terms for malnutrition and wasting syndromes appear in the literature. International consensus groups have labored to provide clear-cut definitions (1, 2, 3). A first important distinction is that cancer is not merely malnutrition or starvation (i.e., defined as a lack of nutrient supply) as the sole cause of depletion of the body reserves. The unqualified term malnutrition is relevant in the instance of deficient food supply, as in medical conditions such as anorexia nervosa. Patients with cancer are affected by a more complex pathophysiology driven by a variable combination of reduced food intake and abnormal metabolism, and this superimposed burden of metabolic alteration is regarded as the essential distinction between cachexia and simple malnutrition (Fig. 87.1) (1, 3). Two terms currently found in the literature, diseaseassociated malnutrition (2) and cachexia (used hereafter in this chapter), can be considered equivalent. Diseaseassociated malnutrition was defined by an international guideline committee constituted to develop a consensus approach to defining the etiology of malnutrition syndromes for adults in the clinical setting (2). These authors defined cancer-associated malnutrition as “a chronic disease or condition that imposes sustained inflammation of a mild to moderate degree.” In the view of many researchers and clinical experts, a primary requirement for the development of cachexia is the presence of an inflammatory process (1, 3, 4, 5, 6). The presence of a chronic inflammatory state seems to account for seemingly disparate aberrations, including changes in the hypothalamic- pituitary axis, dysautonomia, hypermetabolism, oxidative stress, decreased muscle protein synthesis, and increased ubiquitin-proteosome-mediated muscle protein degradation, together with other metabolic changes such as insulin resistance (7, 8, 9, 10, 11, 12, 13). Through its effect on multiple organs and tissues, chronic inflammation leads to the pathophysiologic profile accounting for cachexia.
An international consensus group assembled under the auspices of the European Palliative Care Research Collaborative (EPCRC) developed a conceptual framework for the definition of cancer cachexia (1). This group also underscored the importance of underlying metabolic changes that drive weight loss in the tumor-bearing host.
However, although the EPCRC group acknowledged the importance of inflammation, the group also noted that cachexia may exist without overt systemic inflammation. The elevated energy expenditure and capture of substrates by the tumor was identified as a key catabolic driver. Lieffers et al (14) followed the growth of metastatic tumor burden over time in patients with advanced colorectal cancer in a study using serial computed tomography scans. The mean burden of metastatic disease (0.7 kg) and the high specific metabolic rate of tumor marked a quantitatively important contribution of tumor to whole body basal metabolic rate. Endocrine abnormalities such as insulin resistance, high-dose prolonged corticosteroid therapy (which induces Cushing-like muscle atrophy), and hypogonadism are additional endogenous and exogenous changes potentially contributing to catabolism. Finally, Fearon et al (1) underscored the ability of low levels of physical activity to potentiate catabolic stimuli as an exacerbating factor.
Simple malnutrition is reversed by the provision of food, except when malnutrition is severe enough to induce permanent changes (e.g., growth stunting in children). By contrast, cachexia cannot be fully reversed by conventional nutritional support, and this is regarded as one of the defining characteristics of cachexia syndromes (2). Nutrition supplementation alone only partly reverses or prevents negative energy balance and muscle protein loss in active inflammatory states (15). The efficacy of nutritional intervention may be undermined by the presence of catabolic metabolic abnormalities; however, it is a misconception that nutritional support for the patient with cancer cachexia is futile. Inadequate food intake, in any case, aggravates loss of weight and lean tissue, and adequate feeding may help to limit these losses. Food intake is a primary site of intervention, whether by dietetic counseling, pharmacologic agents intended to stimulate appetite, or artificial nutritional support.
CONTROLS OF ENERGY BALANCE AND METABOLISM IN CANCER CACHEXIA VERSUS HEALTHY INDIVIDUALS
Controls of energy balance and metabolism associated with cancer have many deviations. The involuntary weight loss associated with cancer represents an important failure of the normal control of energy balance. Weight loss is often a presenting symptom and has been long known to predict shortened survival (16). By contrast, energy homeostasis has a high priority in healthy adults, in whom precise metabolic controls help to store food energy optimally or, conversely, to mobilize reserves under appropriate circumstances. A tight coupling of intake with energy expenditure is a mechanism to conserve energy when food supply is limited; in contrast, it also allows for disposing of or storing excess caloric intake. In healthy people, palatable, energy-dense foods retain high incentive value even when immediate energy requirements have been met, and this feature promotes the development of an energy reserve for potential future food shortages. Some individual variation in this response occurs, and the most susceptible individuals exhibit a weak satiety response to fatty meals, a maintained preference for high-fat over low-energy foods in the postingestive satiety period, and a strong hedonic attraction to palatable foods (17).
By contrast to the tight control of energy balance and tendency for energy storage in healthy individuals, patients with cancer lose controls contributing to weight maintenance or gain. Food intake in the patient with cancer may not correlate with degree of weight gain or loss in the usual way (18). Attempts to supplement food
intake by either consultation by a dietitian or nutrition supplementation fail to prevent the progressive weight loss (19). Evidence indicates that deliberate ingestion of supplements is counteracted by reduction in food intake at other meals of the day even in patients with cancer whose intake is less than that required to cover the costs of basal metabolism. When Fearon et al (19) provided an energy-dense nutritional supplement to patients with pancreas cancer, these patients consumed 450 kcal/day of this product but had a net increase of total energy intake of only 68 kcal/day because of decreased intake during other meals of the day. Compensating decreases in oral intake at meals mean that supplementation may be completely ineffective in the worst case or merely inefficient in the best case. The hedonic value of food in general, as well as that of palatable high-energy density foods, is also lost to patients with cancer, and spontaneous ideation about food and food enjoyment disappear (20, 21).
intake by either consultation by a dietitian or nutrition supplementation fail to prevent the progressive weight loss (19). Evidence indicates that deliberate ingestion of supplements is counteracted by reduction in food intake at other meals of the day even in patients with cancer whose intake is less than that required to cover the costs of basal metabolism. When Fearon et al (19) provided an energy-dense nutritional supplement to patients with pancreas cancer, these patients consumed 450 kcal/day of this product but had a net increase of total energy intake of only 68 kcal/day because of decreased intake during other meals of the day. Compensating decreases in oral intake at meals mean that supplementation may be completely ineffective in the worst case or merely inefficient in the best case. The hedonic value of food in general, as well as that of palatable high-energy density foods, is also lost to patients with cancer, and spontaneous ideation about food and food enjoyment disappear (20, 21).
Cachexia also differs from malnutrition in the resulting changes in body composition. During simple starvation, all organs lose mass. In the tumor-bearing state, muscle, skin, bone, and adipose tissue are catabolic, whereas organs such as liver and spleen and many parts of the immune system are anabolic and accumulate protein (14). The anabolic state of the liver exceeds the accumulation of liver protein because much of its anabolism is reflected in an increased production of secretory proteins of the acute phase response (22). During starvation, fat stores are mobilized, and most tissues convert to fat-derived fuels (free fatty acids, ketones) to meet metabolic demands. Use of ketones instead of glucose spares protein catabolism and lean body mass. In contrast, glucose production in cancer cachexia is maintained via gluconeogenesis, thus promoting protein catabolism and facilitating muscle wasting and early lean body mass depletion (23). Increased gluconeogenesis during starvation is transient because hepatic glucose production is displaced by β-oxidation of lipid; however, gluconeogenesis and hepatic glucose production are not suppressed in cancer cachexia (24). Finally, during periods when appetite has been stimulated, weight gain in patients with cancer is often transitory and is associated frequently with gain of fat but not protein (25).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree