Cancer
Cancer, also called malignant neoplasia, refers to a group of more than 100 different diseases that are characterized by deoxyribonucleic acid (DNA) damage that causes abnormal cell growth and development. Malignant cells have two defining characteristics: They can no longer divide and differentiate normally, and they have acquired the ability to invade surrounding tissues and travel to distant sites.
Cancer ranks second to cardiovascular disease as the leading cause of death in the United States. One in four deaths is due to cancer. Some epidemiologists predict that it will outrank cardiovascular disease in the near future. Every year, more than 1 million cancer cases are diagnosed in the United States, and 550,000 people die of cancer-related causes. One-third of these deaths are related to nutrition problems, physical inactivity, obesity, and other lifestyle factors that are preventable in most cases.
Cancer development
The most widely held theory about carcinogenesis involves a three-stage process: initiation, promotion, and progression.
INITIATION
Initiation refers to the damage to or mutation of DNA that occurs when the cell is exposed to an initiating substance or event (such as chemicals, a virus, or radiation) during DNA replication (transcription). Usually, enzymes detect errors in transcription and remove or repair them. Sometimes, however, an error is missed. If regulatory proteins recognize the error and block further division, then the error may be repaired or the cell may self-destruct, a process known as apoptosis. If these proteins miss the error, it becomes a permanent mutation that’s passed on to future generations of cells.
PROMOTION
Promotion involves the mutated cell’s exposure to factors (promoters) that enhance its growth. This exposure may occur shortly after initiation or years later.
Promoters may be hormones such as estrogen, food additives such as nitrates, or drugs such as nicotine. They can affect the mutated cell by altering:
♦ the function of genes that control cell growth and replication
♦ cell response to growth stimulators or inhibitors
♦ intercellular communication.
PROGRESSION
Progression occurs as tumor cells acquire additional mutations, enabling the tumor to invade adjacent tissue, metastasize to distant sites, and become resistant to therapy. This step is irreversible.
Causes
The healthy body is well equipped to defend itself against cancer. Only when the immune system and other defenses fail does cancer prevail.
Evidence suggests that cancer develops from a complex interaction of exposure to carcinogens and accumulated mutations in genes that
control cell growth. Researchers have identified approximately 100 control genes, which fall into four types: proto-oncogenes, tumor-suppressor genes, DNA repair genes, and apoptosis genes.
control cell growth. Researchers have identified approximately 100 control genes, which fall into four types: proto-oncogenes, tumor-suppressor genes, DNA repair genes, and apoptosis genes.
Each of the four types of genes plays a role. When proto-oncogenes mutate, they become oncogenes, leading to uncontrolled cell death growth. Mutated tumor-suppressor genes lose the ability to stop abnormal cell growth. Mutations in DNA repair genes allow damage to go unrepaired, and mutations in apoptosis genes result in damaged cells losing the ability to selfdestruct.
Common causes of acquired genetic damage are viruses, radiation, environmental and dietary carcinogens, and hormones. Other factors that interact to increase a person’s likelihood of developing cancer are age, nutritional status, hormonal balance, and response to stress.
GENETICS
Although cancers may be considered genetic in nature because they result from mutations in genes, only about 10% are inherited. When mutations occur in reproductive cells (germline), damage can be passed on to future generations. This results in a predisposition to develop the cancer.
 The discovery of cancerrelated genes has enhanced the identification, guidance, and management of persons at high risk for common cancers associated with inheritance of cancer-predisposing, genetic mutations. Recent discoveries include:
The discovery of cancerrelated genes has enhanced the identification, guidance, and management of persons at high risk for common cancers associated with inheritance of cancer-predisposing, genetic mutations. Recent discoveries include:♦ Breast and ovarian cancers—Women with a BRCA 1 or BRCA 2 gene alteration have a 36% to 85% lifetime risk of developing breast cancer and a 16% to 60% lifetime risk of developing ovarian cancer.
♦ Colon cancer—In a gene alteration, the helicase-like transcription factor gene (one of the genes that helps stabilize DNA and regulates protein production in the cell) is inactivated, which contributes to the transformation of normal colon cells into cancer cells.
♦ Melanoma—The p16 gene causes some melanomas that run in certain families.
Common characteristics of genetically predisposed cancer include:
♦ early onset of malignant disease
♦ increased incidence of bilateral cancer in paired organs (breasts, adrenal glands, kidneys, and eighth cranial nerve [acoustic neuroma])
♦ increased incidence of multiple primary cancers in nonpaired organs
♦ increased risk of development of a less differentiated tumor
♦ abnormal chromosome complement in tumor cells.
VIRUSES
Viral proto-oncogenes typically contain DNA that’s identical to that of human oncogenes. In animal studies of viral ability to transform cells, some viruses that infect people have demonstrated the potential to cause cancer. For example, the Epstein-Barr virus, which causes infectious mononucleosis, has been linked to Burkitt’s lymphoma and nasopharyngeal carcinoma.
IMMUNOSURVEILLANCE FAILURE
Research suggests that cancer cells develop continually, but the immune system recognizes these cells as foreign and destroys them. This defense mechanism, termed immunosurveillance, has two major components: cell-mediated immune response and humoral immune response. Together these two components interact to promote antibody production, cellular immunity, and immunologic memory. Researchers believe that an intact immune system is responsible for spontaneous regression of tumors. Thus, cancer development is a concern for patients who are immunodeficient or who must take an immunosuppressant.
Cell-mediated immune response
Cancer cells carry cell-surface antigens (specialized protein molecules that trigger an immune response) called tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs). The cellmediated immune response begins when T lymphocytes encounter a TAA or a TSA and become sensitized to it. After repeated contacts, the sensitized T cells release chemical factors called lymphokines, some of which begin to destroy the antigen. This reaction triggers the transformation of a different population of T lymphocytes into “killer T lymphocytes” targeted to cells carrying the specific antigen—in this case, cancer cells.
Humoral immune response
The humoral immune response reacts to a TAA by triggering the release of antibodies from plasma cells and activating the serum-complement system to destroy the antigen-bearing cells. However, an opposing immune factor, a “blocking antibody,” may enhance tumor growth by protecting malignant cells from immune destruction.
Disruption of the immune response
Immunosurveillance isn’t a fail-safe system. If the immune system fails to recognize tumor cells as foreign, the immune response won’t activate. The tumor will continue to grow until
it’s beyond the immune system’s ability to destroy it. In addition to this failure of surveillance, other mechanisms may come into play.
it’s beyond the immune system’s ability to destroy it. In addition to this failure of surveillance, other mechanisms may come into play.
The tumor cells may suppress the immune defenses. The tumor antigens may combine with humoral antibodies to form complexes that essentially hide the antigens from the normal immune defenses. These complexes could also depress further antibody production. Tumors also may change their antigenic “appearance” or produce substances that impair usual immune defenses. The tumor growth factors not only promote the growth of the tumor but also increase the person’s risk of infection. Finally, prolonged exposure to a tumor antigen may deplete the patient’s lymphocytes and further impair the ability to mount an appropriate response.
The patient’s population of suppressor T lymphocytes may be inadequate to defend against malignant tumors. Suppressor T lymphocytes usually assist in regulating antibody production; they also signal the immune system when an immune response is no longer needed. Certain carcinogens, such as viruses or chemicals, may weaken the immune system by destroying or damaging suppressor T cells or their precursors, and subsequently allow for tumor growth.
Theoretically, cancer develops when any of several factors disrupts the immune response:
♦ Aging cells. As cells age, errors in copying genetic material during cell division may give rise to mutations. If the aging immune system doesn’t recognize these mutations as foreign, the mutated cells may proliferate and form a tumor.
♦ Cytotoxic drugs or steroids. These agents decrease antibody production and destroy circulating lymphocytes.
♦ Extreme stress or certain viral infections. These conditions may depress the immune response, thus allowing cancer cells to proliferate.
♦ Suppression of immune system. Radiation, cytotoxic drug therapy, and lymphoproliferative and myeloproliferative diseases (such as lymphatic and myelocytic leukemia) depress bone marrow production and impair leukocyte function.
♦ Acquired immunodeficiency syndrome. This condition weakens the cell-mediated immune response.
♦ Cancer. The disease itself is immunosuppressive. Advanced disease exhausts the immune system, leading to anergy (the absence of immune reactivity).
Risk factors
Many cancers are related to specific environmental or lifestyle factors that predispose a person to develop cancer. Accumulating data suggest that some of these risk factors initiate carcinogenesis, other risk factors act as promoters, and some risk factors initiate and promote the disease process.
AIR POLLUTION
Air pollution has been linked to the development of cancer, particularly lung cancer. Persons living near industries that release toxic chemicals have an increased risk of cancer. Many outdoor air pollutants—such as arsenic, benzene, hydrocarbons, polyvinyl chlorides, and other industrial emissions as well as vehicle exhaust —have been studied for their carcinogenic properties.
Indoor air pollution, such as from cigarette smoke and radon, also poses an increased risk of cancer. In fact, indoor air pollution is considered to be more carcinogenic than outdoor air pollution.
TOBACCO
Cigarette smoking increases the risk of lung cancer more than tenfold over that of nonsmokers by late middle age. Tobacco smoke contains nitrosamines and polycyclic hydrocarbons, two carcinogens that are known to cause mutations. The risk of lung cancer from cigarette smoking correlates directly with the duration of smoking and the number of cigarettes smoked per day. Tobacco smoke is also associated with laryngeal cancer and is considered a contributing factor in cancer of the bladder, pancreas, kidney, and cervix. Research also shows that smoking cessation may decrease a person’s risk of lung cancer.
Although the risk associated with pipe and cigar smoking is similar to that of cigarette smoking, some evidence suggests that the effects are less severe. Smoke from cigars and pipes is more alkaline. This alkalinity decreases nicotine absorption in the lungs and is more irritating to the lungs, so the smoker doesn’t inhale as readily.
Inhalation of secondhand smoke, or passive smoking, by nonsmokers also increases the risk of lung and other cancers. Use of smokeless tobacco, in which the oral tissue directly absorbs nicotine and other carcinogens, is linked to an increase in oral cancers that seldom occur in persons who don’t use the product.
The human papillomavirus (HPV), types 16 and 18, causes 70% of cervical cancer cases. To prevent this disorder, encourage the patient to do the following:
Get vaccinated
Since 2006, the quadrivalent HPV recombinant vaccine (Gardasil) has been available to reduce cervical cancer. This vaccine is recommended for girls and women ages 9 to 26. The vaccine is most effective if given before the patient is sexually active. Remind the patient that Pap tests are still recommended.
Have Pap test screenings
The most effective way to screen for cervical cancer is the Pap test. Screenings should be conducted as follows:
♦ every year or every 2 years using liquidbased Pap tests, beginning approximately 3 years after onset of vaginal intercourse, but no later than age 21
♦ every 2 to 3 years at or older than age 30 for women who have had three consecutive normal Pap test results (more frequent screening may be indicated with certain risk factors)
♦ every 3 years after age 30 (but not more frequently) with either conventional or liquidbased Pap tests, plus the human papillomavirus deoxyribonucleic acid test. Screening may stop for women age 70 and older who have had three consecutive normal Pap tests in 10 years.
Be careful with sexual activity
Having sexual intercourse at a young age increases the risk for contracting HPV. Advise your patient that delaying first intercourse may help reduce this risk. Also, having fewer sexual partners may decrease the risk.
Don’t smoke
Chances of developing cervical cancer can be reduced by not smoking.
ALCOHOL
Alcohol consumption, especially in conjunction with cigarette smoking, is commonly associated with cirrhosis of the liver, a precursor to hepatocellular cancer. The risk of breast and colorectal cancers also increases with alcohol consumption. Possible mechanisms for breast cancer development include impaired removal of carcinogens by the liver, impaired immune response, and interference with cell membrane permeability of the breast tissue. Alcohol stimulates rectal cell proliferation in rats, an observation that may help explain the increased incidence of colorectal cancer in humans who regularly consume alcohol.
Heavy use of alcohol and cigarette smoking synergistically increase the incidence of cancers of the mouth, larynx, pharynx, and esophagus. Alcohol probably acts as a solvent for the carcinogenic substances found in smoke, enhancing their absorption.
SEXUAL BEHAVIOR
Sexual practices have been linked to specific types of cancer. The age of first sexual intercourse and the number of sexual partners are positively correlated with a woman’s risk of cervical cancer. Furthermore, a woman who has had only one sexual partner is at higher risk if that partner has had multiple partners. The suspected underlying mechanism here involves virus transmission, most likely human papillomavirus (HPV). HPV types 6 and 11 are associated with genital warts. HPV is the most common cause of abnormal Papanicolaou smears, and cervical dysplasia is a direct precursor to squamous cell carcinoma of the cervix, both of which have been linked to HPV (especially types 16 and 18). (See Preventing cervical cancer.)
OCCUPATION
Because of exposure to specific substances, certain occupations increase the risk of cancer. People exposed to asbestos, such as insulation installers and miners, are at risk for a type of lung cancer called mesothelioma. Asbestos also may act as a promoter for other carcinogens. Workers involved in the production of dyes, rubber, paint, and beta-naphthylamine are at increased risk for bladder cancer.
ULTRAVIOLET RADIATION
Exposure to ultraviolet B (UVB) radiation can damage the skin and increase the risk of skin cancer development. UVB is known to cause damage to the DNA of skin cells. Specifically, it causes a genetic mutation in the P53 tumorsuppressor gene. Recent evidence shows that
exposure to ultraviolet A (UVA) radiation from sunlamps and tanning booths also contributes to skin cancer development. The damaging effects of UVA are thought to be indirect, occurring as a result of energy transferred through reactive oxygen intermediates (free radicals).
exposure to ultraviolet A (UVA) radiation from sunlamps and tanning booths also contributes to skin cancer development. The damaging effects of UVA are thought to be indirect, occurring as a result of energy transferred through reactive oxygen intermediates (free radicals).
Most basal cell carcinomas can be prevented through lifestyle changes. Teach your patient to do the following:
Avoid the midday sun
Sunlight is strongest between 10 a.m. and 4 p.m. Outdoor activities should be scheduled for other times of the day, even in winter or when it’s cloudy. Advise your patient that sunlight is more intense when it reflects off water, sand, and snow.
Use sunscreen year-round
Sunscreens don’t filter out all harmful UV radiation, but they’re helpful for sun protection. Advise your patient to wear a broad-spectrum sunscreen with a sun protection factor (SPF) of at least 15 when he goes outside, year-round. He should use about 1 ounce—the amount that fits in his palm—to cover his entire body, including lips, ears, and the backs of hands and neck. Sunscreen should be applied 20 to 30 minutes before sun exposure and reapplied every 2 hours throughout the day, as well as after swimming or exercising.
Wear protective clothing
Sunscreens shouldn’t be relied on as the sole means of sun protection. It’s important to also wear tightly woven clothing that covers the arms and legs and a broad-brimmed hat rather than a baseball cap or visor. Sunglasses that provide full protection from both UVA and UVB rays should also be worn.
Be careful with medications
Some common prescription and over-thecounter drugs make skin more sensitive to sunlight. These include antibiotics; certain cholesterol, high blood pressure, and diabetes medications; ibuprofen (Advil, Motrin); and the acne medication isotretinoin (Accutane).
Perform regular skin checks
Encourage your patient to examine his skin often for new growths or changes in existing moles, freckles, bumps, and birthmarks, including on the scalp, ears, and buttocks, and to be vigilant about checking for recurring tumors.
Get enough vitamin D
This vitamin may help lower the risk of certain cancers. Although it’s normally produced by sunlight on the skin, many experts recommend getting the daily requirement of vitamin D through food or supplements.
The amount of exposure to ultraviolet radiation also correlates with the type of cancer that develops. For example, cumulative exposure to ultraviolet radiation is associated with basal and squamous cell skin cancer, and severe episodes of burning and blistering at a young age are associated with melanoma. (See Preventing basal cell carcinoma.)
IONIZING RADIATION
Ionizing radiation (such as X-rays) is associated with acute leukemia, thyroid, breast, lung, stomach, colon, and urinary tract cancers as well as multiple myeloma. Low doses can cause DNA mutations and chromosomal abnormalities, and large doses can inhibit cell division. This damage can directly affect carbohydrate, protein, lipid, and nucleic acids (macromolecules), or it can act on intracellular water to produce free radicals that damage the macromolecules.
Ionizing radiation can also enhance the effects of genetic abnormalities. For example, it increases the risk of cancer in people with a genetic abnormality that affects DNA repair mechanisms. Other compounding variables include the area and percentage of the body exposed, the person’s age, hormonal balance, prescribed drugs, and preexisting or concurrent conditions.
HORMONES
Hormones—specifically the sex steroid hormones estrogen, progesterone, and testosterone —have been implicated as promoters of breast, endometrial, ovarian, and prostate cancers.
Estrogen, which stimulates the proliferation of breast and endometrial cells, is considered a promoter for breast and endometrial cancers. Prolonged exposure to estrogen, as in women with early menarche and late menopause, increases the risk of breast cancer. Likewise,
long-term use of estrogen replacement therapy without progesterone supplementation for menopausal symptoms increases a woman’s risk of endometrial cancer. Progesterone may play a protective role, counteracting estrogen’s stimulatory effects.
long-term use of estrogen replacement therapy without progesterone supplementation for menopausal symptoms increases a woman’s risk of endometrial cancer. Progesterone may play a protective role, counteracting estrogen’s stimulatory effects.
The recommendations listed below from the American Cancer Society focus on nutrition and physical activity prevention measures.
♦ Eat a variety of healthful foods, with an emphasis on plant sources.
♦ Eat five or more servings of a variety of vegetables and fruits each day. Choose whole grains in preference to processed (refined) grains and sugars.
♦ Limit consumption of red meats, especially processed ones and those high in fat.
♦ Choose foods that can help you maintain a healthful weight.
♦ Adopt a physically active lifestyle.
♦ Adults: Engage in at least moderate activity for 30 minutes or more on 5 or more days of the week; 45 minutes or more of moderate to vigorous activity on 5 or more days per week may further reduce the risk of breast and colon cancer.
♦ Children and adolescents: Engage in at least 60 minutes/day of moderate-to-vigorous physical activity at least 5 days/ week.
♦ Maintain a healthful weight throughout life.
♦ Balance caloric intake with physical activity.
♦ Lose weight if currently overweight or obese.
♦ Don’t smoke, and limit or abstain from consumption of alcoholic beverages.
The male sex hormones stimulate the growth of prostatic tissue. However, research fails to show an increased risk of prostate cancer in men who take exogenous androgens.
DIET
Numerous aspects of diet are linked to an increase in cancer, including:
♦ obesity (in women only, possibly related to production of estrogen by fatty tissue), which is linked to a suspected increased risk of endometrial cancer
♦ high consumption of dietary fat, which is linked to endometrial, breast, prostate, ovarian, and rectal cancers
♦ high consumption of smoked foods, salted fish or meats, and foods containing nitrites, which may be linked to gastric cancer
♦ naturally occurring carcinogens (such as hydrazines and aflatoxin) in foods, which are linked to liver cancer
♦ carcinogens produced by microorganisms stored in foods, which are linked to stomach cancer
♦ diet low in fiber (which slows transport through the gut), which is linked to colorectal cancer.
The American Cancer Society has developed specific nutritional guidelines for cancer prevention. (See American Cancer Society recommendations for cancer prevention.)
Pathophysiologic changes
Cancer’s characteristic features are uncontrollable proliferation of cells and independent spread from a primary site (site of origin) to other tissues where it establishes secondary foci (metastasis). This spread occurs through circulation in the blood or lymphatic fluid, by unintentional transplantation from one site to another during surgery, and by local extension. Thus, cancer cells differ from normal cells in terms of cell size, shape, number, differentiation, and purpose or function. In addition, cancer cells can travel to distant tissues and organ systems. (See Cancer cell characteristics.)
CELL GROWTH
Typically, each of the billions of cells in the human body has an internal clock that tells the cell when it’s time to reproduce. Mitotic reproduction occurs in a sequence called the cell cycle. Normal cell division occurs in direct proportion to cells lost, thus providing a mechanism for controlling growth and differentiation. These controls are absent in cancer cells, and cell production exceeds cell loss. Consequently, cancer cells enter the cell cycle more frequently and at different rates. They’re most commonly found in the synthesis and mitosis phases of the cell cycle, and they spend very little time in the resting phase.
Normal cells reproduce at a rate controlled through the activity of specific control or regulator genes (called proto-oncogenes when they function normally). These genes produce proteins that act as on-and-off switches. There’s no generalized control gene; different cells respond to specific control genes. The P53 and c-myc genes are two examples of control genes: P53 is
a tumor-suppressor gene that can stop DNA replication if the cell’s DNA has been damaged; c-myc helps initiate DNA replication and if it senses an error in DNA replication, it can cause the cell to self-destruct.
a tumor-suppressor gene that can stop DNA replication if the cell’s DNA has been damaged; c-myc helps initiate DNA replication and if it senses an error in DNA replication, it can cause the cell to self-destruct.
Hormones, growth factors, and chemicals released by neighboring cells or by immune or in-flammatory cells can affect control gene activity. These substances bind to specific receptors on the cell membranes and send out signals causing the control genes to stimulate or suppress cell reproduction. Examples of hormones and growth factors that affect control genes include:
♦ erythropoietin, which stimulates red blood cell (RBC) proliferation
♦ epidermal growth factor, which stimulates epidermal cell proliferation
♦ insulin-like growth factor, which stimulates fat and connective tissue proliferation
♦ platelet-derived growth factor, which stimulates connective tissue cell proliferation.
Substances released by injured or infected nearby cells or by cells of the immune system also affect cellular reproduction. For example, interleukin, released by immune cells, stimulates cell proliferation and differentiation. Interferon, released from virus-infected and immune cells, may affect the cell’s rate of reproduction.
Additionally, cells that are close to one another appear to communicate with each other through gap junctions (channels through which ions and other small molecules pass). This communication provides information to the cell about the neighboring cell types and the amount of space available. The nearby cells send out physical and chemical signals that control the reproduction rate. For example, if the area is crowded, the nearby cells will signal the same type of cells to slow or cease reproduction, thus allowing the formation of only a single layer of cells. This feature is called densitydependent growth inhibition.
In cancer cells, the control genes fail to function normally. The control may be lost or the gene may become damaged. An imbalance of growth factors may occur, or the cells may fail to respond to the suppressive action of the growth factors. Any of these mechanisms may lead to uncontrolled cellular reproduction.
One striking characteristic of cancer cells is that they fail to recognize the signals emitted by nearby cells about available tissue space. Instead of forming only a single layer, cancer cells continue to accumulate in a disorderly array.
The loss of control over normal growth is termed autonomy. This independence is further evidenced by the ability of cancer cells to break off and travel to other sites.
Cancer cell characteristics
Cancer cells, which undergo uncontrolled cellular growth and development, typically exhibit these characteristics:
♦ Vary in size and shape
♦ Undergo abnormal mitosis
♦ Function abnormally
♦ Don’t resemble the cell of origin
♦ Produce substances not usually associated with the original cell or tissue
♦ Aren’t encapsulated
♦ Can spread to other sites
CELL DIFFERENTIATION
Usually, cells become specialized during development. That is, the cells develop highly individualized characteristics that reflect their specific structure and functions in their corresponding tissue. For example, all blood cells are derived from a single stem cell that differentiates into RBCs, white blood cells (WBCs), platelets, monocytes, and lymphocytes. As the cells become more specialized, their reproduction and development slow down. Eventually, highly differentiated cells become unable to reproduce and some— skin cells, for example— are programmed to die and be replaced.
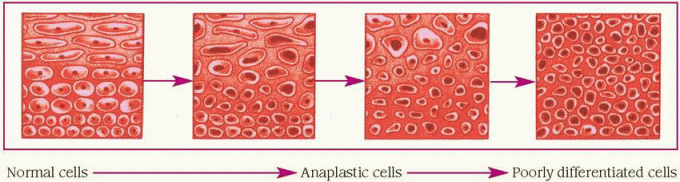
Cancer cells lose the ability to differentiate— that is, they enter a state, called anaplasia, in which they no longer appear or function like the original cell. (See Understanding anaplasia, page 18.)
Anaplasia occurs in varying degrees. The less the cells resemble the cell of origin, the more anaplastic they’re said to be. As the anaplastic cells continue to reproduce, they lose the typical characteristics of the original cell.
Some anaplastic cells begin functioning as another type of cell, possibly becoming a site for hormone production. For example, small-cell lung cancer cells often produce antidiuretic hormone, which is produced by the hypothalamus but stored in and secreted by the posterior pituitary gland.
When anaplasia occurs, cells of the same type in the same site exhibit many different shapes and sizes. Mitosis is abnormal and chromosome defects are common.
INTRACELLULAR CHANGES
The abnormal and uncontrolled cell proliferation of cancer cells is associated with numerous changes within the cancer cell itself. These changes affect the cell membrane, cytoskeleton, and nucleus.
Cell membrane
The cell membrane is a thin, dynamic, semipermeable structure that separates the cell’s internal environment from its external environment. It consists of two layers of lipid molecules (called the lipid bilayer) with protein molecules attached to or embedded in each layer. The bilayer is composed of phospholipids, glycolipids, and other lipids, such as cholesterol.
The protein molecules help stabilize the structure of the membrane and participate in the transport and exchange of material between the cell and its environment. Large glycoproteins, called fibronectin, are responsible for holding the cells in place and maintaining the specific arrangement of the receptors to allow for the exchange of material.
In the cancer cell, fibronectin is defective or is broken down as it’s produced, thus affecting the organization, structure, adhesion, and migration of the cells. Some of the other proteins and glycolipids are also absent or altered. These changes affect the density of the receptors on the cell membrane and the cell’s shape. Communication between the cells becomes impaired, response to growth factors is enhanced, and recognition of other cells is diminished. The result is uncontrolled growth.
Permeability of the cancer cell membrane is also altered. During its uncontrolled, rapid proliferation, the cancer cell has a much greater metabolic demand for nutrients to sustain its growth.
During normal development, cell division can occur only when the cells are anchored to nearby cells or to extracellular molecules via anchoring junctions. In cancer cells, anchoring junctions need not be present. Thus, they continue to divide and can metastasize.
Disruption or blockage of gap junctions interferes with intercellular communication. This may be the underlying mechanism by which cancer cells continue to grow and migrate, forming layers of undifferentiated cells, even in a crowded environment.
Cytoskeleton
The cytoskeleton is composed of protein filament networks including actin and microtubules. Usually, actin filaments exert a pull on the extracellular organic molecules that bind cells together. Microtubules control cell shape, movement, and division. In cancer cells, the functions of these components are altered. Additionally, cytoplasmic components are fewer and abnormally shaped. Less cellular work occurs because of a decrease in endoplasmic reticulum and mitochondria.
Nucleus
In cancer cells, nuclei are pleomorphic, meaning enlarged and of various shapes and sizes. They’re also highly pigmented and have larger and more numerous nucleoli than normal. The nuclear membrane is typically irregular and commonly has projections, pouches, or blebs, and fewer pores. Chromatin (uncoiled chromosomes) may clump along the outer areas of the nucleus. Breaks, deletions, translocations, and abnormal karyotypes (chromosome shape and number) are common changes in the chromosomes. The chromosome defects seem to stem from the increased mitotic rate in cancer cells. The appearance of the mitotic cancer cell under light microscopy is commonly described as atypical and bizarre.
TUMOR DEVELOPMENT AND GROWTH
Typically, a long time passes between the initiating event and the appearance of cancer. During this time, the cancer cells continue to grow, develop, and replicate, each time undergoing successive changes and further mutations.
How fast a tumor grows depends on specific characteristics of the tumor and its host.
Tumor growth needs
For a tumor to grow, an initiating event or events must cause a mutation that will transform the normal cell into a cancer cell. After the initial event, the tumor continues to grow only if available nutrients, oxygen, and blood supply are adequate and the immune system fails to recognize or respond to the tumor.
Location and blood supply
Two important tumor characteristics affecting growth are tumor location and available blood supply. The location determines the originating cell type, which in turn determines the cell cycle time. For example, epithelial cells have a shorter cell cycle than that of connective tissue cells. Thus, tumors arising from epithelial cells (such as squamous cell carcinomas and adenocarcinomas) grow more rapidly than tumors arising from connective tissue cells (such as fibrosarcomas).
Tumors need an available blood supply to provide nutrients and oxygen for continued growth and to remove wastes; however, a tumor larger than 1 to 2 mm in size has typically outgrown its available blood supply. Some tumors secrete tumor angiogenesis factors, which stimulate the formation of new blood vessels, to meet the demand.
The degree of anaplasia also affects tumor growth. Remember that the more anaplastic the tumor’s cells, the less differentiated the cells and the more rapidly they divide.
Many cancer cells also produce their own growth factors. Numerous growth factor receptors are present on the cell membranes of rapidly growing cancer cells. This increase in receptors, in conjunction with the changes in the cell membranes, further enhances cancer cell proliferation.
Host characteristics
Several important characteristics of the host affect tumor growth. These characteristics include age, sex, overall health status, and immune system function.
 Age is an important factor affecting tumor growth. Relatively few cancers are found in children. Yet the incidence of cancer correlates directly with increasing age. This correlation suggests that numerous or cumulative events are necessary for the initial mutation to continue, eventually forming a tumor.
Age is an important factor affecting tumor growth. Relatively few cancers are found in children. Yet the incidence of cancer correlates directly with increasing age. This correlation suggests that numerous or cumulative events are necessary for the initial mutation to continue, eventually forming a tumor.Certain cancers are more prevalent in one sex than in the other. For example, sex hormones influence tumor growth in breast, endometrial, cervical, and prostate cancers. Researchers believe that the hormone sensitizes the cell to the initial precipitating factor, thus promoting carcinogenesis.
Overall health status also is an important host characteristic. As tumors obtain nutrients for growth from the host, they can alter normal body processes and cause cachexia. Conversely, if the person is nutritionally depleted, tumor growth may slow. Chronic tissue trauma also has been linked with tumor growth because healing involves increased cell division. The more rapidly cells divide, the greater the likelihood of mutations.
SPREAD OF CANCER
Between the initiating event and the emergence of a detectable tumor, some or all of the mutated cells may die. The survivors, if any, reproduce until the tumor reaches a diameter of 1 to 2 mm. New blood vessels form to support continued growth and proliferation. As the cells further mutate and divide more rapidly, they become more undifferentiated. The number of cancerous cells soon begins to exceed the number of normal cells. Eventually, the tumor mass extends, spreading into local tissues and invading the surrounding tissues. When the local tissue is blood or lymph, the tumor can gain access to the circulation. Once access is gained, tumor cells that detach or break off travel to distant sites in the body, where they can survive and form a new tumor in that secondary site. This process is called metastasis.
Dysplasia
Not all cells that proliferate rapidly go on to become cancerous. Throughout a person’s life span, various body tissues experience periods of benign rapid growth such as during wound healing. Sometimes changes in the size, shape, and organization of the cells lead to a condition called dysplasia.
Exposure to chemicals, viruses, radiation, or chronic inflammation causes dysplastic changes that may be reversed by removing the initiating stimulus or treating its effects. However, if the stimulus isn’t removed, precancerous or dysplastic lesions can progress and give rise to cancer. For example, actinic keratoses, thickened patches on the skin of the face and hands
of people exposed to sunlight, are associated with the development of skin cancer. Removal of the lesions and use of sunblock help minimize the risk that the lesions will progress to skin cancer. Knowledge about precancerous lesions and promoter events provide the rationale for early detection and screening as important preventive measures.
of people exposed to sunlight, are associated with the development of skin cancer. Removal of the lesions and use of sunblock help minimize the risk that the lesions will progress to skin cancer. Knowledge about precancerous lesions and promoter events provide the rationale for early detection and screening as important preventive measures.
Localized tumor
Initially, a tumor remains localized. Recall that cancer cells communicate poorly with nearby cells. As a result, the cells continue to grow and enlarge, forming a mass or clumps of cells. The mass exerts pressure on the neighboring cells, blocking their blood supply, and subsequently causing their death.
Invasive tumor
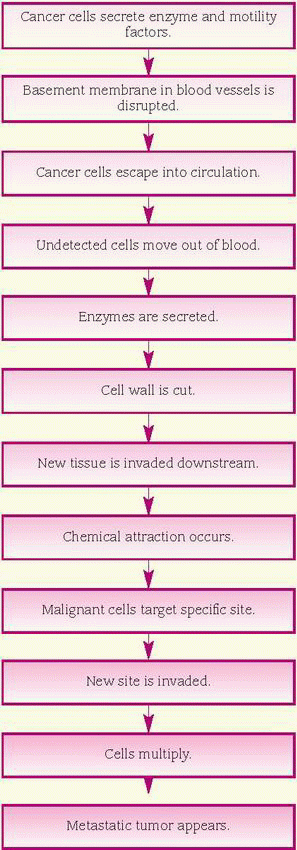
Invasion is growth of the tumor into surrounding tissues. It’s the first step in metastasis. Five mechanisms are linked to invasion: cellular multiplication, mechanical pressure, lysis of nearby cells, reduced cell adhesion, and increased motility. Experimental data indicate that the interaction of all five mechanisms is necessary for invasion.
By their very nature, cancer cells multiply rapidly (cellular replication). As they grow, they exert pressure on surrounding cells and tissues, which eventually die because their blood supply has been cut off or blocked (mechanical pressure). Loss of mechanical resistance leads the way for the cancer cells to spread along the lines of least resistance and occupy the space once filled by the dead cells.
Vesicles on the cancer cell surface contain a rich supply of receptors for laminin, a complex glycoprotein that’s a major component of the basement membrane, a thin sheet of noncellular connective tissue upon which cells rest. These receptors permit the cancer cells to attach to the basement membrane, forming a bridgelike connection (lysis of nearby cells). Some cancer cells produce and excrete powerful proteolytic enzymes; other cancer cells induce normal host cells to produce them. These enzymes, such as collagenases and proteases, destroy the normal cells and break through their basement membrane, enabling the cancer cells to enter.
Reduced cell adhesion also is seen with cancer cells. As discussed in the section on intracellular changes, reduced cell adhesion likely results when the cell-stabilizing glycoprotein fibronectin is deficient or defective.
Cancer cells also secrete a chemotactic factor that stimulates and increases motility. Thus, the
cancer cells can move independently into adjacent tissues and into the circulation, and then on to a secondary site. Finally, cancer cells develop fingerlike projections called pseudopodia that facilitate cell movement. These projections injure and kill neighboring cells and attach to vessel walls, enabling the cancer cells to enter.
cancer cells can move independently into adjacent tissues and into the circulation, and then on to a secondary site. Finally, cancer cells develop fingerlike projections called pseudopodia that facilitate cell movement. These projections injure and kill neighboring cells and attach to vessel walls, enabling the cancer cells to enter.
Metastatic tumor
Metastatic tumors are those in which the cancer cells have traveled from the original or primary site to a second or more distant site. Most commonly, metastasis occurs through the blood vessels and lymphatic system. Tumor cells also can be transported from one body location to another by external means, such as carriage on instruments or gloves during surgery.
Hematogenous metastasis
Invasive tumor cells break down the basement membrane and walls of blood vessels, and the tumor sheds malignant cells into the circulation. Most of the cells die, but a few escape the host defenses and the turbulent environment of the bloodstream. From here, the surviving mass of tumor cells, called a tumor cell embolus, travels downstream and commonly lodges in the first capillary bed it encounters. For example, blood from most organs next enters the capillaries of the lungs, which are the most common sites of metastasis.
Once lodged, the tumor cells develop a protective coat of fibrin, platelets, and clotting factors to evade detection by the immune system. Then they become attached to the epithelium, ultimately invading the vessel wall, interstitium, and parenchyma of the target organ. (See How cancer metastasizes.) To survive, the new tumor develops its own vascular network and may ultimately spread again.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree