Calmodulin is a highly conserved, ubiquitous calcium-binding protein in cells that modulates a wide variety of cellular reactions. Calmodulin contains four calcium-binding (“EF-hand”) domains that contain a high proportion of glutamate and aspartate (acidic) residues. These calcium-binding domains are highly conserved and consist of approximately 30 to 40 amino acid residues in a helix-loop-helix structure known as an EF-hand (Figure 32-1). Calcium-binding domains usually occur in pairs and are also present in other cellular calcium-binding proteins, such as troponin C in muscle and calbindin-D9K in the intestine. Although many calcium-binding proteins contain calmodulin-like motifs, some other calcium-binding proteins, such as the vitamin K–dependent proteins that contain γ-carboxyglutamate (Gla) residues and the annexins, do not contain EF-hand domains. Calcium acts as a second messenger by increasing cytosolic calcium, binding to Ca2+-binding protein (i.e., calmodulin) to create a conformational change that alters cell activity, with subsequent removal of the Ca2+. Almost all the calcium within cells is bound within organelles such as the endoplasmic reticulum (ER), the nucleus, and other membrane-bound compartments. Consequently, the free calcium in the cell cytosol is significantly lower than in the extracellular space, with a 10,000-fold chemical gradient for Ca2+ across cell membranes. Very small changes in the release of Ca2+ from intracellular sites or in its transport across the cell membrane will cause a large increase in cytosolic Ca2+ concentration. Changes in intracellular Ca2+ concentrations in response to cell-surface binding of peptide hormones or growth factors (first message) can act as a second messenger to elicit a variety of cellular activity, as summarized in Box 32-1. Cytosolic Ca2+ can bind to cellular Ca2+-binding proteins, including calmodulin, a ubiquitous cytosolic protein that can activate cellular kinases and other enzymes, and troponin C, a muscle protein that is bound to actinomycin contractile fibers. Binding of Ca2+ to calcium-dependent proteins such as calmodulin causes a conformational change in the protein that can then directly or indirectly alter cellular activity, as illustrated in Figure 32-2. Phospholipase A2 (PLA2) is an enzyme that releases fatty acids from the second carbon group of glycerol and has a strong, predetermined fold that generates a rather immobile cavity for Ca2+ binding. Ca2+ is needed to hold the phosphate group of the phospholipid substrate in a suitable location for hydrolysis of the sn-2 ester linkage. Arachidonic acid is derived from membrane phospholipids by the action of PLA2 and is the rate-limiting step in prostaglandin synthesis, as described in Chapter 18. Phospholipases A2 include several protein families with common enzymatic activity with two Ca2+-dependent families, the secreted and cytosolic PLA2. Two other families include Ca2+-independent PLA2 (iPLA2) and lipoprotein-associated PLA2s (lp-PLA2), also known as platelet-activating factor acetylhydrolase (PAF-AH) (Burke and Dennis, 2009). Calpains are Ca2+-dependent proteinases that contain calmodulin-like domains with EF-hand binding sites. Calpains are nonlysosomal, intracellular proteases present in both the cytoplasm and the nucleus and are active at neutral pH. Calpain proteinase complexes consist of an approximate catalytic and regulatory subunit. Progressive binding of Ca2+ to the calpain complex is linearly related to the dissociation of the two subunits, which reaches completion when all eight Ca2+-binding sites of calpain (four per subunit) are occupied, whereas the activity of the catalytic subunit itself also is enhanced with greater physiological concentrations of Ca2+ (Reverter et al., 2001). There is increasing evidence that ubiquitous calpains participate in a variety of cellular processes including remodeling of cytoskeletal and membrane attachments, signal transduction pathways, and apoptosis. The activity of calpains is tightly controlled by the endogenous inhibitor calpastatin (Goll et al., 2003). In mammals and plants, calpain plays an essential role in development. Research suggests that calpains are involved in the cell degeneration processes that characterize numerous disease conditions linked to dysfunctions of cellular Ca2+ homeostasis, and especially in neurodegeneration. Atypical calpains may be associated with disease, such as calpain-10 that is linked to type 2 diabetes (Bertipaglia and Carafoli, 2007). Two enzymes involved in the blood-clotting cascade, prothrombin and factor X, require calcium for their activation. Calcium binds to a protein site on prothrombin for its activation and to the phospholipid or the protein–phospholipid complex of factor X. In addition, calcium binding is essential for activation of protein S (Rezende et al., 2004), which is an important anticoagulant in the body. Annexins are a family of Ca2+– and phospholipid-binding proteins present in all eukaryotes. Annexins are defined as being capable of binding negatively charged phospholipids in a Ca2+-dependent reversible manner and must contain a 70–amino acid repeat sequence called an annexin repeat. Annexins are found both inside and outside of cells and act as membrane scaffold proteins involved in membrane–membrane and membrane–cytoskeleton interactions. They are involved in the mediation of Ca2+-regulated processes including endocytosis, exocytosis, cytoskeletal regulation, and membrane conductance and organization (Fatimathas and Moss, 2010; Rescher and Gerke, 2004). Several pathological conditions may be modified by the annexins in the progression of cancer, diabetes, and the autoimmune disorder, antiphospholipid syndrome. Inorganic phosphate has several important roles in many biological processes, including cell growth, cell signaling, nucleic acid synthesis, energy metabolism, membrane function, and bone mineralization. Phosphate also helps to maintain normal acid–base balance (pH) by acting as a buffer. Additionally, the phosphorus-containing molecule 2,3-diphosphoglycerate (2,3-DPG) binds to hemoglobin in red blood cells and influences oxygen delivery to the tissues of the body (Knochel, 2006). An acute decrease in plasma phosphate or a negative phosphate balance can lead to serious disease, including myopathy, cardiac dysfunction, abnormal neutrophil function, platelet dysfunction, and fragile red cell membrane. Chronically low phosphate balance will lead to impaired bone mineralization (rickets or osteomalacia). In contrast, elevated plasma phosphate will lead to secondary hyperparathyroidism, as is common in patients with chronic kidney disease. Neutral molecules can pass more readily across cell membranes than charged molecules. Phosphorylation of substrates can result in the metabolic trapping of the phosphorylated compounds within a cell and is considered a crucial event for intestinal absorption of riboflavin (Gastaldi et al., 2000) and vitamin B6. Nonspecific phosphatases in the intestine hydrolyze the 5′-phosphate of each vitamin, allowing passage into the enterocyte via a nonsaturable, passive absorption process. After absorption, the vitamers (any chemical compound exhibiting vitamin-like activity) can be phosphorylated and thus retained. Coenzymes with catalytic functions in enzyme reactions transfer chemical groups to other molecules. Some coenzymes contain AMP moieties contributed by ATP in the coenzyme synthetic pathway, such as nicotinamide adenine dinucleotide (phosphate) [NAD(P)], whereas others are phosphate esters of vitamin precursors. Coenzymes are discussed in more detail in Chapters 24, 25, and 26. Creatine phosphate is a high-energy phosphate ester that is stored in muscle to be used during exercise as a reservoir of available energy. Creatine phosphate is used to replenish ATP levels by transfer of the phosphate group from creatine phosphate to ADP in a reaction catalyzed by creatine kinase. (See Chapter 20 for more detail about the role of creatine phosphate in exercising muscle.) IP3 couples receptor activation at the plasma membrane to Ca2+ release from intracellular stores. Phosphatidylinositol (PtdIns) in the cell membrane is converted by a phosphorylation reaction (PtdIns kinase) into the polyphosphatidylinositols: phosphatidylinositol 4-phosphate (PIP) and phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2). Activation of a cell surface receptor activates a G protein in the cell membrane that in turn activates phospholipase C, which cleaves PI(4,5)P2 to form IP3 and diacylglycerol. Diacylglycerol can be further cleaved to yield arachidonic acid, which can be used to synthesize prostaglandins and other signaling molecules, or it can activate protein kinase C, which is a Ca2+-dependent enzyme. (See Chapters 18 and 19 for more detail about these signaling processes.) Many protein molecules have two or more slightly different conformations that can alter their function. A common mechanism employed by the cell to control the shape of certain proteins involves covalent modification by transfer of a phosphate group from ATP to a serine, threonine, or tyrosine residue in the protein, forming a covalent linkage. The alternate phosphorylation and dephosphorylation of proteins via protein kinases and phosphatases is an important cellular control mechanism. (See Chapters 12 and 19 for more discussion of the role of phosphorylation in the regulation of enzyme activities). In addition, ATP-driven phosphorylation results in conformational changes in membrane-bound proteins that act as pumps to drive the influx or efflux of ions across the cell membrane (e.g., Na+,K+-ATPase pump) (see Chapter 34, Figure 34-2). The skeleton is an important reservoir for minerals in the body; it consists of 70% mineral, 20% collagen, 8% water, and 2% noncollagenous protein. Approximately 99% of the body’s calcium, 85% of the phosphate, 70% of magnesium, and about 50% of sodium are found in bone (Driessens and Verbeeck, 1990). The mineral component of bone is largely calcium and phosphate. In the body, calcium is found mainly as the calcium phosphate compound hydroxyapatite. Forty-seven percent of skeletal weight is dry, fat-free bone, with 26% of the dry, fat-free bone weight contributed by calcium. Osteonectin is the most abundant noncollagenous protein in bone (Robey and Boskey, 2008). It accounts for approximately 20% to 25% of the total noncollagenous protein in compact bone and has a high affinity for binding calcium and hydroxyapatite. It is also called secreted protein acidic and rich in cysteine, sometimes abbreviated SPARC or basement membrane-40, BM-40. It is expressed in many mammalian tissues including muscle, brain, adipose, testes, kidney, skin, bone, and cartilage (Robey and Boskey, 2008). Osteonectin promotes osteoblast proliferation and survival and is critical for the maintenance of bone mass. It is also increased in wound healing, angiogenesis, tumor growth, and metastasis. Vitamin K is a cofactor in the γ-glutamyl carboxylation pathway, a posttranslational conversion of specific glutamate residues into Gla residues (see Chapter 28). Three vitamin K–dependent proteins, osteocalcin, matrix Gla protein, and protein S, are found in the bone. Osteocalcin is a highly conserved 5.7-kDa bone matrix protein found in bone, dentin, and exoskeleton and is synthesized by mature osteoblasts (Hauschka et al., 1989). Also called bone Gla protein, this molecule undergoes vitamin K–dependent γ carboxylation (Gla), which is necessary for conformational changes of the protein and binding capacity to hydroxyapatite. It regulates bone formation and activity of osteoclasts. Serum levels of osteocalcin are used as a biochemical marker of bone formation. However, mice lacking osteocalcin (knockout mouse) have an accelerated rate of bone formation (Ducy et al., 1996), suggesting a more complex role of osteocalcin. In addition, osteocalcin has been implicated as a hormone regulating energy metabolism (Lee et al., 2007) and has been shown to regulate beta cell proliferation and insulin secretion and sensitivity in animal models. Matrix Gla protein (MGP) is a 15-kDa, vitamin K–dependent noncollagenous protein originally isolated from bone, but is predominantly produced by vascular smooth muscle cell and chondrocytes. MGP is a potent inhibitor of vascular calcification, and MGP knockout (−/−) mice die within 6 to 8 weeks because of rupture of arteries (Luo et al., 1997). In humans, calcification is associated with decreased circulating MGP levels, because MGP has high affinity for hydroxyapatite. Protein S is a 70-kDa vitamin K–dependent anticoagulant Gla protein. Whereas protein S is synthesized by the osteoblast and a deficiency of protein S is associated with osteopenia (Maillard et al., 1992), the primary risk factor associated with protein S deficiency is thrombosis. To maintain homeostasis and supply the mineral needs of the body, calcium and phosphate absorption by the intestine and reabsorption by the kidney, and bone turnover are coordinately regulated by the hormones 1,25(OH)2D (also called calcitriol), parathyroid hormone (PTH), calcitonin and fibroblast growth factor 23 (FGF23) as summarized in Figure 32-3 and Box 32-2. PTH is a peptide hormone that is produced by the chief cells of the parathyroid gland and acts on certain cells through PTH receptors on the cell surface. The biologically active form of PTH is a single-chain polypeptide of 84 amino acids; however, only the first 31 amino acids of PTH are essential for biological activity. The parathyroid gland contains the extracellular calcium-sensing receptor (CaSR) that acts as a sensor of plasma ionized calcium levels (Thakker et al., 2010; dePaula and Rosen, 2010). Decreased ionized calcium in the blood is detected by the calcium sensor and leads to an increase in PTH secretion; conversely, secretion is inhibited by an increase in blood calcium. The rapid negative feedback regulation of PTH production is thus largely regulated by plasma calcium. Bone and kidney are the primary target organs for PTH. The peptide hormone interacts with specific receptors on the plasma membrane of bone cells (osteoblasts) and tubular kidney cells to stimulate cAMP production. Cyclic AMP acts as a second messenger to activate certain enzymes, such as protein kinases, which trigger a cascade of biochemical events that ultimately result in expression of the physiological actions of PTH. Increased plasma PTH concentrations are detected by renal PTH receptors, causing the kidneys to rapidly increase the rate of renal calcium reabsorption (which decreases urinary calcium loss) and decrease the rate of phosphate reabsorption (which increases urinary phosphate loss). PTH also increases the activity of the renal 25-hydroxyvitamin D 1α-hydroxylase that converts the biologically inactive 25-hydroxyvitamin D [25(OH)D] into the active hormonal form of vitamin D, 1,25(OH)2D. There are no PTH receptors in the intestine, but the PTH-mediated increase in circulating 1,25(OH)2D leads to an increase in intestinal calcium and phosphate absorption. Also, despite the fact that PTH plays an important role in bone resorption, no PTH receptors are found on the osteoclasts (Gardella et al., 2010). PTH produces a bone-resorbing effect by stimulating expression of RANKL (receptor activator of nuclear factor kappa B [RANK] ligand) by osteoblasts and decreasing osteoprotegerin production by preosteoblast cells in bone (Figure 32-4). These factors work with RANK, a receptor for RANKL that is present on osteoclasts. Receptor activation by RANKL promotes osteoclast differentiation and activation into mature active bone-resorbing osteoclasts (Kearns et al., 2008). These three distinct but coordinated actions of PTH maintain calcium homeostasis by increasing calcium release from bone, reducing the renal clearance of calcium, and increasing absorption in the intestine. Hyperphosphatemia, which would result from the accompanying increased intestinal absorption and skeletal release of phosphorus, is prevented by the phosphaturic effect of PTH on the kidneys. In contrast to chronic high PTH that acts to resorb bone, lower dose intermittent PTH that is used in the treatment of osteoporosis and contains amino acids 1 to 34 (teriparatide) will stimulate bone formation (Dempster et al., 1993). 1,25(OH)2D likely plays many roles in the body and classically acts on cells through an intracellular receptor protein called the vitamin D receptor (VDR) that is found in many different tissues. In addition, a 1,25(OH)2D–membrane-associated rapid response to steroid-binding protein (1,25D3-MARRS) has been found on plasma membranes, and it appears to regulate the rapid nongenomic aspects of 1,25(OH)2D action (Nemere et al., 2004). However, the physiological importance of these rapid vitamin D–mediated effects on cells has yet to be determined. When activated by 1,25(OH)2D, the nuclear VDR interacts with specific gene promoter regions in DNA and affects transcription of these vitamin D–specific genes, such as increasing expression of the enterocyte membrane calcium channel (TRPV6, also called CaT1) (Wood et al., 2001) and calbindin-D9K, a 9-kDa intracellular Ca2+-binding protein that facilitates the absorption of calcium (Bronner, 2009). 1,25(OH)2D also increases expression of a number of other proteins that are involved with other functions of vitamin D, such its immunological or antiproliferative effects (Wood et al., 2004). In addition, 1,25(OH)2D increases the apical membrane Na+-dependent phosphate transporter that facilitates phosphate absorption in the brush border membrane of the enterocyte. Osteoblasts also have vitamin D receptors, and the effect of 1,25(OH)2D on bone is to increase expression of RANKL by osteoblastic cells (see Figure 32-4). Receptor activation by RANKL leads to osteoclast differentiation, activation and survival, thereby promoting differentiation into osteoclasts and bone resorption (Maes and Kronenberg, 2010). However, this effect of 1,25(OH)2D has only been shown with supraphysiological levels, and at physiological doses it suppresses PTH-induced RANKL expression and bone resorption (Suda et al., 2003) (see Chapter 31 for additional information on 1,25(OH)2D). Calcitonin, synthesized by the C cells (previously termed the parafollicular cells) of the thyroid gland, is a polypeptide containing 32 amino acids, almost all of which are needed for biological activity. A rise in plasma Ca2+ is the strongest calcitonin secretagogue. When blood Ca2+ increases acutely, there is a parallel increase in calcitonin secretion, whereas an acute drop in plasma Ca2+ causes a decrease in calcitonin secretion. Calcitonin acts in opposition to PTH and lowers blood calcium levels. The best studied action of calcitonin, which appears to occur generally throughout all mammalian species, is to inhibit osteoclast activity (de Paula and Rosen, 2010). The plasma membrane of osteoclasts has calcitonin receptors that respond to calcitonin by increasing cAMP production, which in turn mediates the actions of calcitonin, including inhibition of the movement of osteoclasts. Inactivation of calcitonin occurs primarily in the kidney. Aside from the osteoclast calcitonin receptor, these receptors are found in other cells, such as monocytes, kidney, brain, pituitary, placenta, prostate, testis, lung, and lymphocytes (Martin et al., 2010). Calcitonin is used as a drug to treat diseases associated with high rates of bone resorption and hypercalcemia (Table 32-1), such as Paget disease and osteoporosis, to reduce vertebral fracture. Calcitonin also has been shown to reduce cartilage degradation in osteoarthritic patients (Karsdal et al., 2010) and has analgesic properties to inhibit bone pain (Knopp et al., 2005). TABLE 32-1 Causes and Clinical Consequences of Hypocalcemia and Hypercalcemia A major advance in understanding phosphate homeostasis was accomplished with the identification of FGF23 as a novel hormone that lowers blood phosphate levels (Kuro-o, 2010). When phosphate is in excess, FGF23 is secreted from osteocytes in bone, a process that is regulated by two bone proteins: phosphate-regulating gene with homology to endopeptidases on the X chromosome (PHEX) and dentin matrix protein 1 (DMP1) (see Figure 32-3). FGF23 acts on the kidney to suppress expression of the sodium-phosphate cotransporters (NaPi-2a, NaPi-2c) which facilitate the active reabsorption of phosphorus. Diminished expression of NaPi-2a and NaPi-2c occurs either directly or indirectly through stimulation of PTH activity, which increases urinary phosphate excretion (Bergwitz and Jüppner, 2010). In addition, FGF23 reduces plasma 1,25(OH)2D levels by suppressing synthesis of 1α-hydroxylase and increasing catabolism by increasing 24-hydroxylase activity. The reduction in 1,25(OH)2D levels results in a reduced phosphate intestinal absorption (and calcium absorption), thereby inducing a negative phosphate balance. FGF23 requires Klotho, a transmembrane protein, as a co-receptor for high affinity binding to FGF receptors. More specifically, FGF23 binds the Klotho–FGF receptor complex with much higher affinity than the FGF receptor alone. There are hereditary disorders or tumor-induced osteomalacia that exhibit inappropriately high plasma FGF23 levels (and low 1,25(OH)2D) and are characterized by phosphate wasting and impaired bone mineralization (Table 32-2). In contrast, defects in either FGF23 or Klotho are associated with phosphate retention and a premature aging syndrome. The causes and outcomes of hyperphosphatemia are outlined in Table 32-2. Several other phosphaturic peptides, such as secreted frizzled-related protein 4 (sFRP4), matrix extracellular phosphoglycoprotein, and fibroblast growth factor 7 (FGF7), have been shown to inhibit renal phosphate reabsorption and have a pathogenic role in several hypophosphatemic disorders. FGF23 and sFRP4 may be regulated by dietary phosphorus intake. Determination of levels of FGF23 and other phosphaturic peptides (phosphatonins) may improve the diagnosis and prognosis of various diseases association with abnormal mineral metabolism including chronic kidney disease. TABLE 32-2 Causes and Clinical Consequences of Hypophosphatemia and Hyperphosphatemia
Calcium and Phosphorus
Chemical Properties of Calcium
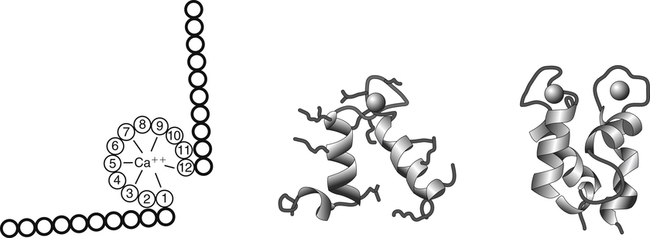
A commonly found calcium-binding motif in proteins is called the EF-hand structure. The EF-hand is composed of two perpendicular 10– to 12–amino acid alpha helices with a 12-residue calcium binding loop region (helix-loop-helix). Calcium ions bind within the calcium binding loop to oxygen ligands provided by amino acids 1, 3, 5, 7, 9, and 12. In most EF-hand proteins the residue at position 12 is a glutamate, which contributes both of its side-chain oxygens for calcium coordination. EF-hand elements usually occur in pairs and are found in a large number of calcium-binding proteins, such as calmodulin and calbindin D.
Physiological and Metabolic Functions of Calcium and Phosphate
Biological Functions Of Calcium
Calcium as a Second Messenger
Increased Cytosolic Ca2+
Calcium-Dependent Trigger Proteins in Cells
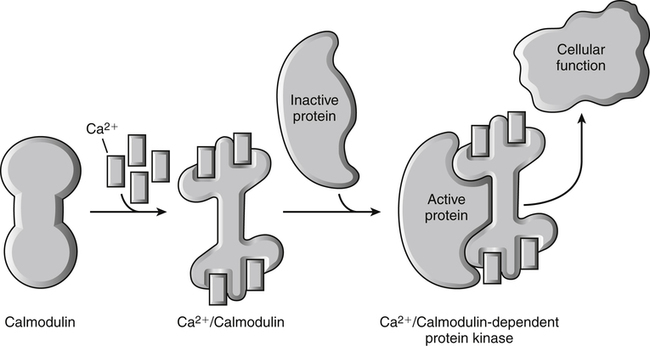
Calcium ions (Ca2+) bind to calmodulin, a ubiquitous cellular calcium-binding protein, resulting in a conformational change in this protein. The Ca2+-dependent alteration of calmodulin structure exposes a region of the protein that can now bind to and activate a calmodulin-dependent target enzyme. The activation of the enzyme subsequently results directly or indirectly in a change in cellular function. The Ca2+ signal is turned off by lowering the cytosolic Ca2+ concentration, which can be achieved by sequestering free Ca2+ or pumping the Ca2+ out of the cell via plasma membrane Ca2+-ATPase pumps. Also see Box 32-1 for cellular function of intracellular Ca2+. (Modified from Cantley, L. [2009]. Signal transduction. In W. F. Boron & E. L. Boupaep (Eds.), Medical physiology [2nd ed., p. 62]. Philadelphia: Saunders.)
Role of Calcium in Activation of Other Proteins
Phospholipase A2
Calpains
Blood-Clotting Enzymes
Annexins
Biological Functions of Phosphate
Metabolic Trapping of Substrates
Nucleotides, Creatine Phosphate, and Other Phosphoesters
Signaling Molecules: Cyclic AMP, Cyclic GMP, and Inositol Triphosphate
Reversible Covalent Modification of Proteins
Calcium and Phosphorus as Components of Mineralized Tissue
Calcium-Binding Proteins in Bone
Osteonectin
Gla Proteins
Hormonal Regulation of Calcium and Phosphate Metabolism
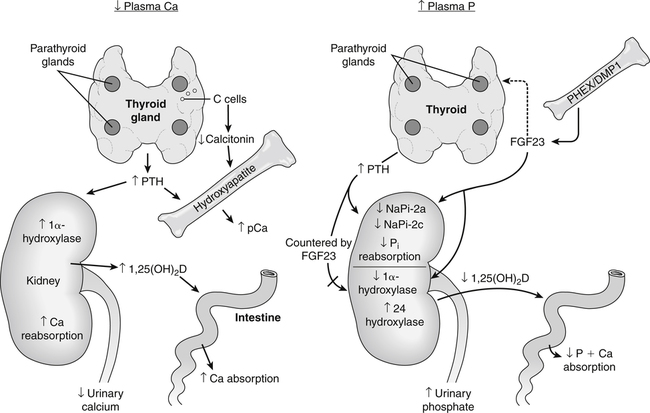
Calcium and phosphate homeostasis is maintained by the coordinated actions of the intestine, kidney, and bone. A fall in plasma ionic Ca2+ level is detected by a calcium-sensor protein in the parathyroid glands. This causes an increased secretion of parathyroid hormone (PTH) that then acts on receptors in the kidney and in bone osteoblasts. The renal effect of PTH results in an immediate increase in renal calcium reabsorption and a decrease in renal phosphate reabsorption. PTH also has a delayed effect on calcium and phosphate absorption by stimulating the activity of the renal 1α-hydroxylase that converts inactive 25-hydroxyvitamin D [25(OH)D] to the active vitamin D metabolite 1,25-dihydroxyvitamin D [1,25(OH)2D]. In addition, 1,25(OH)2D can also promote bone resorption by stimulating the production of osteoclasts. Fibroblast growth factor 23 (FGF23) is the most important regulator of phosphate renal reabsorption and acts to suppress sodium–phosphate cotransporter type-2a (NaPi-2a) and NaPi-2c and either alone or in conjunction with PTH acts to increase urinary phosphate excretion. In addition, FGF23 acts to lower plasma phosphorus by countering the actions of PTH on 1α-hydroxylase and 1,25(OH)2D production and increasing the activity of 24 hydroxylase, which ultimately leads to lower intestinal phosphorus absorption. DMP1, Dentin matrix protein 1; pCa, plasma calcium; PHEX, phosphate-regulating gene with homologies to endopeptidases on the X chromosome.
Parathyroid Hormone
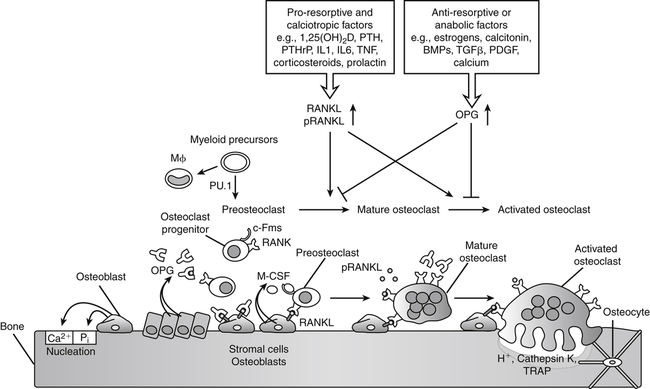
Transcription factor PU.1 is required for development of the osteoclast and macrophage (M) lineages from a common myeloid precursor. Macrophage-colony stimulating factor (M-CSF) produced by osteoblast cells acts upon the receptor c-Fms and the osteoclast progenitor is converted to a preosteoclast. Signaling through the membrane-bound receptor activator of nuclear factor-κB (RANK) promotes further differentiation of preosteoclasts to mature osteoclasts that secrete protons (H+, acid) and proteolytic enzymes to resorb bone such as tartrate-resistant acid phosphatase (TRAP) and cathepsin K. RANK signaling is induced by receptor activator of nuclear factor-κB ligand (RANKL), present on osteoblasts, bone marrow stromal cells, lymphocytes, and possibly in a soluble form in plasma (pRANKL). The antagonist osteoprotegerin (OPG) competes with RANK for RANKL binding, thereby functioning as a negative regulator of osteoclast differentiation, activation, and survival. RANKL expression is induced by proresorptive factors (e.g., cytokines TNFα and IL1, PTH, and 1,25(OH)2D) that stimulate osteoclastogenesis. Conversely, osteoprotegerin is induced by factors that block bone catabolism and promote anabolic effects (e.g., bone morphogenic protein, BMP; transforming growth factor-β, TGFβ; platelet-derived growth factor, PDGF) to regulate bone resorption. PTHrP, Parathyroid hormone–related protein. (Modified from Maes, C., & Kronenberg, H. M. [2010]. Bone development and remodeling. In J. L. Jameson & L. J. De Groot [Eds.], Endocrinology [p. 1118]. Philadelphia: Saunders.)
25-HyDroxyvitamin D and 1,25-Dihydroxyvitamin D
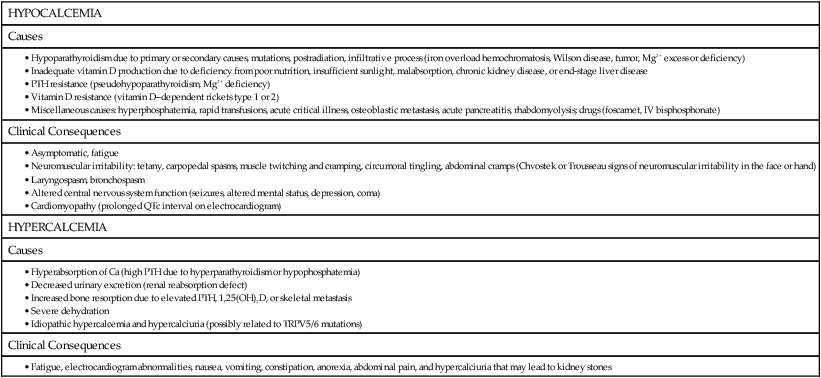
Calcitonin
Fibroblast Growth Factor 23



