Breast Lesions in Men and Children
Needle core biopsy and fine needle aspiration of the male breast are uncommon procedures, and they are even less frequent in children. A review of fine needle aspirations of the breast performed in three large academic programs in the United States from 1990 to 2000 revealed that about 4% of 14,026 specimens were obtained from men (1). The most frequent diagnoses were gynecomastia and carcinoma. A pathology group servicing several community hospitals in the Netherlands received 26 core biopsy specimens of the male breast between 1993 and the end of 2002, or 2.6 specimens per year (2). The diagnoses were reported as gynecomastia, benign, or carcinoma.
BENIGN PROLIFERATIVE LESIONS OF THE MALE BREAST
Gynecomastia is the most common clinical and pathologic abnormality of the male breast. Mammary enlargement may be result of a discrete, nodular increase in subareolar tissue, or a diffuse accumulation of tissue. Both breasts are affected in the majority of patients at all ages. Gynecomastia is a frequent adverse side effect of some medications used to treat prostatic hyperplasia (3) and may occur in men who receive antiretroviral treatment for HIV (4). The initial clinical signs of gynecomastia are breast enlargement and a palpable mass that may be accompanied by pain or tenderness. The palpable mass is located in the central subareolar region in most patients. Carcinoma arising in gynecomastia can usually be detected as a localized, asymmetric area of firmness.
Mammography is helpful for distinguishing between gynecomastia and carcinoma and may identify carcinoma developing in gynecomastia (5). Two mammographic patterns associated with gynecomastia are a dendritic, retroareolar density with prominent radial extensions into the breast and a triangular subareolar density lacking radiating extensions. Ultrasound has also proven to be useful in the diagnosis of gynecomastia.
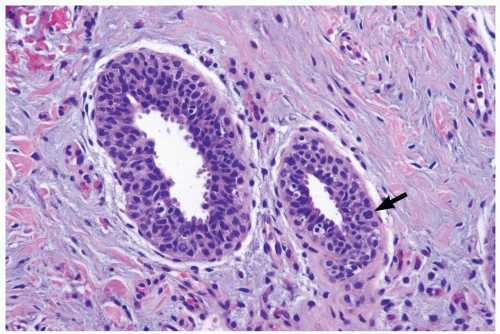
The microscopic features of gynecomastia are similar, regardless of etiologic factors (6,7). Three phases of proliferative change have been described. Florid gynecomastia, seen ordinarily soon after onset, is characterized by prominent epithelial proliferation in ducts, typically with a micropapillary structure (Fig. 27.1). Usually present is concomitant myoepithelial cell hyperplasia. The increased amount and cellularity of periductal stroma are accompanied by prominent vascularity, edema, and a round cell infiltrate. Intermediate gynecomastia has florid and fibrous components. It constitutes a transitional phase in maturation of the lesion (Fig. 27.2). Fibrous or inactive gynecomastia typically occurs after the lesion has been present for 6 months or longer. The epithelial proliferation tends to be less conspicuous than in the florid phase but prominent hyperplasia can persist (Fig. 27.3). The stroma is more collagenous, with less edema, and there is reduced vascularity. Pseudoangiomatous hyperplasia of the stroma may be found in any phase of gynecomastia (Fig. 27.4).
The cytologic features and growth pattern of the epithelial proliferation in ducts may be atypical. This occurs most often in the florid phase. Atypical features are the focal development of fenestrated and solid growth patterns and papillary proliferation in which cytologically atypical cells appear to overgrow the dimorphic population that characterizes florid gynecomastia (Fig. 27.5). Mitotic activity is variable, but it may be pronounced in ducts exhibiting atypical hyperplasia. Nuclear estrogen receptor immunoreactivity is present in the epithelial cells in most examples of gynecomastia. Uncommon proliferative epithelial changes found in gynecomastia include lobule formation, pseudolactational hyperplasia, apocrine metaplasia, cysts, and squamous metaplasia (Fig. 27.6).
Gynecomastia-like hyperplasia of the female breast is composed of stromal and ductal proliferative elements that duplicate the appearance of true gynecomastia in men (8,9). The majority of the patients are premenopausal. The lesion is either palpable or it is detected as an asymmetric density on mammography (10). The radiologic findings
are not specific, and, in some instances, no lesions may be detected on the mammogram (8,10).
are not specific, and, in some instances, no lesions may be detected on the mammogram (8,10).
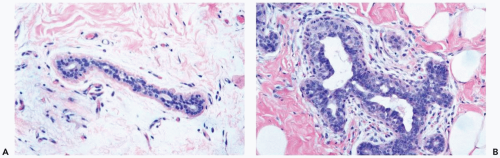 Figure 27.1 Gynecomastia, florid. A. A normal duct in the breast of a 75-year-old man. B. Gynecomastia with hyperplasia of myoepithelial cells and edema of the periductal stroma. |
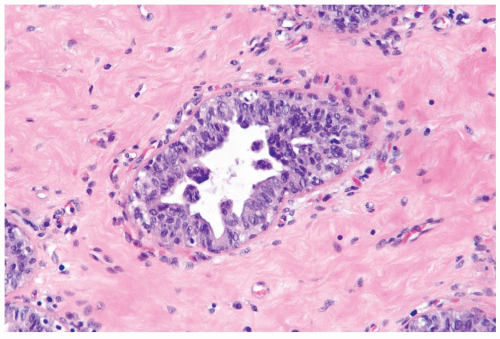 Figure 27.3 Gynecomastia, inactive. This focus features micropapillary epithelial hyperplasia and a mild increase in periductal vascularity. The periductal stroma is collagenized. |
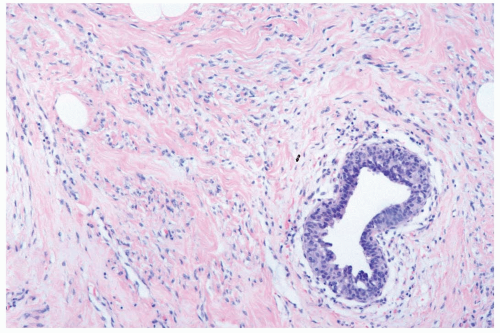 Figure 27.4 Gynecomastia. Pseudoangiomatous hyperplasia of the stroma surrounds a duct with myoepithelial hyperplasia. |
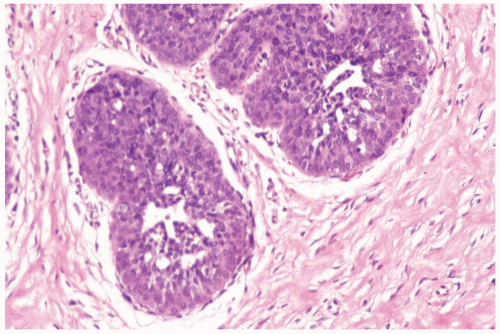 Figure 27.5 Gynecomastia with atypical duct hyperplasia. The proliferation is almost entirely epithelial with a minimal myoepithelial component. |
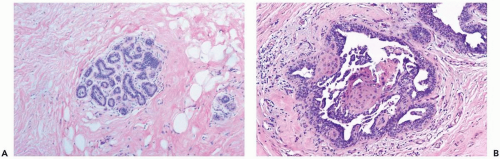 Figure 27.6 Gynecomastia. A. Lobule formation in a male breast. B. Florid gynecomastia with squamous metaplasia in hyperplastic duct epithelium. |
Intraductal papillomas can arise in the male breast (11). The usual presenting symptom is nipple discharge that is bloody or blood tinged. Microscopic examination reveals an orderly papillary structure composed of cuboidal or columnar cells. Because of the relatively high proportion of papillary carcinomas of the male breast, all male papillary tumors should be carefully evaluated pathologically. Approximately 5% of the reported examples of florid papillomatosis of the nipple have been in men (12). Nearly half of these lesions have contained carcinoma.
Fibroepithelial tumors of the male breast usually arise in a background of gynecomastia. Some lesions classified as fibroadenoma or phyllodes tumor are probably nodular foci of gynecomastia (13,14). Most phyllodes tumors in men have been histologically and clinically benign. Some of the male patients with fibroepithelial lesions had been treated with estrogens, resulting in concomitant gynecomastia with lobular differentiation (14).
Other benign conditions in the male breast include duct ectasia (15) and fibrocystic changes (16), both often coexistent with gynecomastia. Myofibroblastoma is a relatively common benign mesenchymal tumor of the male breast (see Chapter 23).
CARCINOMA OF THE MALE BREAST
Breast carcinoma in men accounts for not more than 1% of all breast carcinomas (17). A straight-line relationship exists between incidence and age among men (18). This differs from female breast carcinoma, which has a less steep slope of incidence after age 50 (19). A study of male breast carcinoma cases in Florida revealed an increase from 56 in 1985 to 132 in 2000, representing a significant rise in incidence (20). The age-specific incidence was highest in men older than age 70.
The majority of male breast carcinomas are located centrally in a retroareolar position. The cumulative risk for bilaterality is 3% or less. Carcinoma is found in about 75% of male patients with a mass and bloody discharge. Serous discharge alone may indicate intraductal carcinoma (21). Age at diagnosis averages about 60 years, but breast carcinoma has been diagnosed in adult males at virtually all ages. Almost 90% of male breast carcinomas express estrogen receptor (22). HER2/neu expression is found in male breast carcinoma less frequently than in female breast carcinoma (23).
Mammograms of men with breast carcinoma typically reveal distinct lesions with invasive margins that contrast sharply with the surrounding fatty tissue (24,25). Cystic papillary carcinoma produces a discrete, round mass that may contain calcifications (26). Microcalcifications are found in 9% to 30% of male breast carcinomas studied mammographically (27,28).
Approximately 85% of male mammary carcinomas are of the ordinary infiltrating duct variety (29,30) (Fig. 27.7). An intraductal component is found in 35% to 50% of male and 75% of female infiltrating duct carcinomas (29




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree