Chapter 13 Questions 1. A patient underwent a lumbar puncture and cerebrospinal fluid (CSF) was obtained. No additional clinical history was provided. Based on the image in Figure 13-1, the patient would most likely have which one of the following? B. Bacterial meningitis. C. Aseptic meningitis. D. Parasitic infection. E. Lymphoma. 2. A 79-year-old woman with a history of breast carcinoma and smoking presented with multiple brain masses. Figure 13-2 shows the cells in the CSF obtained by lumbar puncture. These findings are most consistent with which one of the following diagnoses? A. Metastatic breast carcinoma. B. Metastatic lung small cell carcinoma. C. Metastatic melanoma. D. Metastatic lymphoma. E. Metastatic lung adenocarcinoma. 3. The CSF specimen in Figure 13-3, obtained by lumbar puncture from a 5-year-old boy, most likely represents which one of the following entities? B. Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). C. Acute lymphoblastic leukemia (ALL). D. Medulloblastoma. E. Bacterial meningitis. 4. A 45-year-old man with a history of renal transplantation presented to his urologist for routine follow-up. The image in Figure 13-4 is from his voided urine cytology specimen. Which one of the following is the best diagnosis in this case? A. Positive for malignant cells; urothelial carcinoma. B. Positive for malignant cells; renal clear cell (conventional) carcinoma. C. Negative for malignant cells; polyomavirus infection. D. Negative for malignant cells; hyaline inclusion bodies. E. Negative for malignant cells; renal tubular cells. 5. A 50-year-old man with no significant past medical history presented with microhematuria. The image in Figure 13-5 is from his voided urine cytology specimen. Which one of the following is the best diagnosis in this case? B. Hyaline inclusion body. C. Corpora amylacea. D. Trichomoniasis. E. Calcium oxalate crystal. 6. A 39-year-old woman presented with symptoms of a urinary tract infection. A voided urine specimen was sent to the laboratory for further analysis. Several large cells were noted in the specimen, similar to the one seen in the center of Figure 13-6. Which one of the following is the best diagnosis for this type of cell? B. Squamous cell. C. Renal tubular cell. D. Basal cell. E. Umbrella cell. 7. A 92-year-old woman presented with gross hematuria. A voided urine specimen was sent for evaluation, and cells seen in Figure 13-7 were identified. Which one of the following is the best diagnosis for this specimen? A. Negative for malignant cells; umbrella cells. B. Positive for malignant cells; urothelial carcinoma. C. Negative for malignant cells; instrumentation effect. D. Positive for malignant cells; lymphoma. E. Negative for malignant cells; vaginal contamination. 8. A 69-year-old man with microhematuria had a voided urine specimen submitted for evaluation. Numerous crystals were seen as in Figure 13-8. Which one of the following is the best diagnosis for these crystals? A. Calcium oxalate crystals; pathologic. B. Triple phosphate crystals; nonpathologic. C. Uric acid crystals; pathologic. D. Cystine crystals; nonpathologic. E. Tyrosine crystals; nonpathologic. 9. As part of routine follow-up, a 45-year-old man with a history of lung transplantation underwent bronchoscopy and a bronchoalveolar lavage (BAL) was obtained. The BAL had the cells seen in Figure 13-9. Which one of the following is the best diagnosis based on this case? A. Positive for malignant cells; adenocarcinoma. B. Positive for malignant cells; lymphoma. C. Negative for malignant cells; benign bronchial cells. D. Negative for malignant cells; pulmonary alveolar macrophages. E. Negative for malignant cells; benign squamous cells. 10. As a routine follow-up, a 45-year-old man with a history of lung transplantation had a BAL. Which one of the following is the best diagnosis based on the findings in Figure 13-10? B. Candida sp. C. Aspergillus sp. D. Cytomegalovirus. E. Pneumocystis carinii. 11. A patient presented with a pleural effusion with numerous mesothelial cells and eosinophils. Which one of the following is the most common cause of an eosinophilic effusion? B. Drug reaction. C. Parasitic infection. D. Rheumatoid arthritis. E. Systemic lupus erythematosus. 12. A 42-year-old man presented with pneumonia. Imaging studies showed a pulmonary infiltrate and a minute, ill-defined, pleural-based thickening. The patient underwent a BAL. Based on Figure 13-11, the patient likely has which one of the following? B. Mesothelioma. C. Pulmonary hemorrhage. D. Aspiration pneumonia. E. Asbestos exposure. 13. A 39-year-old woman with human immunodeficiency virus–acquired immunodeficiency syndrome (HIV-AIDS) presented with fever, cough, and pulmonary infiltrates. She underwent a BAL. Based on Figure 13-12, which one of the following is the best diagnosis? B. Mucormycosis. C. Histoplasmosis. D. Pneumocystis infection. E. Cryptococcosis. 14. An 89-year-old man with shortness of breath and symptoms of pneumonia had a chest x-ray that demonstrated a small pleural effusion. The types of cells seen in the aspiration are shown in Figure 13-13. Which one of the following is the best diagnosis? B. Malignant; adenocarcinoma. C. Negative; mostly histiocytes. D. Malignant; lymphoma. E. Negative; mostly mesothelial cells. 15. Figure 13-14 shows the cells in a pleural effusion from a 70-year-old man. Which one of the following is the best diagnosis? B. Benign; histiocytes. C. Malignant; mesothelioma. D. Benign; mesothelial cells. E. Malignant; adenocarcinoma. 16. A voided urine specimen was submitted for evaluation. No additional clinical history was provided. Which one of the following is seen in Figure 13-15? B. Cystine crystals. C. Tyrosine crystals. D. Calcium oxalate crystals. E. Sulfadiazine crystals. 17. A patient underwent a lumbar puncture and CSF was obtained. No additional clinical history was provided. Based on the image in Figure 13-16, the patient would most likely be considered to have which one of the following entities? B. Traumatic tap. C. Glioblastoma multiforme (GBM). D. Signet ring adenocarcinoma. E. Old hemorrhage. 18. A 50-year-old woman who is HIV positive presented with headaches and fevers. The findings shown in Figure 13-17 of the CSF obtained by lumbar puncture are most consistent with which one of the following diagnoses? B. Cryptococcus. C. Chondrocytes. D. Contamination (starch). E. Parasitic infection. 19. The CSF specimen in Figure 13-18 was obtained by lumbar puncture from a 5-year-old boy. The findings are most consistent with which one of the following diagnoses? B. Bacterial meningitis. C. Aseptic meningitis. D. Medulloblastoma. E. Small cell carcinoma. 20. A voided urine specimen was submitted for evaluation. No additional clinical history was provided. Based on the image in Figure 13-19, which one of the following is the best interpretation? A. White blood cell cast; no clinical significance. B. White blood cell cast; clinically significant. C. Red blood cell cast; no clinical significance. D. Red blood cell cast; clinically significant. E. Renal tubular epithelial cell casts; clinically significant. 21. A voided urine specimen was submitted for evaluation. No additional clinical history was provided. Based on the image in Figure 13-20, which one of the following is the best interpretation? A. White blood cell cast; no clinical significance. B. White blood cell cast; clinically significant. C. Red blood cell cast; no clinical significance. D. Red blood cell cast; clinically significant. E. Renal tubular epithelial cell cast; clinically significant. 22. A voided urine specimen was submitted for evaluation. No additional clinical history was provided. Which one of the following is seen in Figure 13-21? B. Cystine crystals. C. Tyrosine crystals. D. Calcium oxalate crystals. E. Sulfadiazine crystals. 23. A voided urine specimen was submitted for evaluation. No additional clinical history was provided. Based on Figure 13-22, which one of the following is the best interpretation? A. White blood cell cast; no clinical significance. B. White blood cell cast; clinically significant. C. Red blood cell cast; no clinical significance. D. Red blood cell cast; clinically significant. E. Renal tubular epithelial cell casts; clinically significant. 1. A. Normal findings. Major points of discussion ■ Normal CSF is sparsely cellular with only rare lymphocytes and monocytoid cells. ■ Neutrophils in a CSF may be a “red herring” if numerous red blood cells from peripheral blood are present as a result of a traumatic tap. ■ The differential diagnosis for a CSF with numerous lymphocytes includes a reactive process and lymphoma. ■ A reactive process generally has a polymorphous population of lymphoid cells, including smaller and larger cells, as well as plasma cells. Lymphoma tends to have a more monomorphic population of lymphocytes. ■ Flow cytometry may be necessary to confirm the diagnosis of lymphoma, especially when a low-grade lymphoma composed of smaller lymphocytes is suspected.1 2. A. Metastatic breast carcinoma. Major points of discussion ■ Lymphoid cells occur singly and do not form tight cohesive clusters. Numerous lymphoid cells are often accompanied by lymphoglandular bodies, which are pale-blue cytoplasmic fragments of different shapes and approximately the size of the red blood cells present in the background. ■ Nuclear molding is evident in clusters and is defined as cohesive cell nuclei conforming to the contour of adjacent cells. ■ Nuclear molding is seen in small cell carcinomas and medulloblastomas. ■ Small cell carcinoma affects adults, whereas medulloblastoma affects children. ■ Carcinomas present as single cells and cell clusters. ■ Conspicuous nucleoli are not a feature of small cell carcinoma but may be seen in adenocarcinoma. ■ Small cell carcinomas can arise in various organs. Clinical history, not morphology, is useful in determining the primary site.1 3. A. Aseptic meningitis. Major points of discussion ■ Lymphoid cells occur singly. ■ Cell type (epithelial, lymphoid, or other), distribution (single or clusters), and age (child or adult) aid in narrowing the differential diagnosis. ■ The differential diagnosis for a cellular CSF with lymphoid cells also includes a reactive process and lymphoma. ■ A polymorphous population of lymphocytes is suggestive of a reactive process. ■ A monotonous population of lymphocytes is likely to represent lymphoma. Confirmation with ancillary studies, such as flow cytometry, may be necessary for low-grade lymphomas composed of smaller lymphoid cells that lack prominent nucleoli. ■ Medulloblastoma affects the pediatric population. ALL affects young children between the ages of 2 and 7 years and has a second peak later in adulthood.1 4. A. Positive for malignant cells; urothelial carcinoma. Major points of discussion ■ Decoy cells are infected by human polyomavirus, also known as BK virus. ■ Decoy cells are seen in both immunosuppressed and immunocompetent patients. ■ The chromatin pattern is helpful in distinguishing between urothelial carcinoma and human polyomavirus–infected cells. ■ The chromatin in urothelial carcinoma tends to be coarse. ■ The nucleus is smudgy or glassy in a decoy cell.1 5. A. Granular cast. Major points of discussion ■ Casts in the urine occur as long tubular structures and corpora amylacea are round. ■ Granular casts are relatively common in urine specimens and usually have no clinical significance. ■ It is important to distinguish casts from parasites. Casts have a homogeneous granular appearance, whereas some internal structures may be seen in parasitic worms. ■ Not to be mistaken for viral inclusions, hyaline inclusion bodies are red structures of varying sizes present in the cytoplasm of urothelial cells. ■ Trichomonas may be difficult to identify due to its small size and relatively pale staining nucleus. On low magnification, the organisms resemble amorphous debris, but the nucleus and characteristic pear shape are better appreciated on higher magnification. Inflammation and a “dirty”-appearing background provide clues to the diagnosis.1 6. A. Endometrial cell. Major points of discussion ■ Umbrella cells and squamous cells can be similar in size and represent the largest cells in urine specimens. ■ Renal tubular cells are the smallest epithelial cells in urine specimens. ■ The two types of cells that are multinucleated in urine specimens include umbrella cells and multinucleated giant cells. ■ Multinucleated giant cells are typically present in patients with a history of urothelial carcinoma treated with bacillus Calmette–Guérin. The cytoplasm of these cells is less granular than that of umbrella cells. ■ Umbrella cells can have a single nucleus or multiple nuclei.1 7. A. Negative for malignant cells; umbrella cells. Major points of discussion ■ Malignant urothelial cells occur singly and/or in clusters. ■ Umbrella cells and squamous cells are the largest cells in urine specimens, but their low nuclear/cytoplasmic ratios differentiate them from malignant cells. ■ Coarse chromatin is useful in discerning urothelial carcinoma from decoy cells, which have smudgy chromatin. ■ Clusters of urothelial cells may be seen in patients who have undergone catheterization or instrumentation (e.g., cystoscopy). Calculi in the urinary tract can also lead to shedding of urothelial clusters in urine specimens. ■ In addition to the cytological features, a clinical history of calculi or cystoscopy is useful in determining the origin of cell clusters.1 8. A. Calcium oxalate crystals; pathologic. Major points of discussion ■ Most crystals have no clinical significance. ■ Cystine and tyrosine crystals are suggestive of underlying disease. ■ Crystals of the same compound can take on different shapes and sizes. ■ The presence of crystals in a urine specimen is often related to pH and temperature. ■ A specimen may have rare or numerous crystals.1,,2 9. A. Positive for malignant cells; adenocarcinoma. Major points of discussion ■ A few types of cells, including bronchial cells and squamous cells, can be present in a BAL, but pulmonary alveolar macrophages are the most common cell type. ■ Macrophages typically do not form clusters and occur as single cells. ■ The low nuclear/cytoplasmic ratio, rather than the large size of macrophages (relative to adjacent inflammatory or red blood cells), is helpful in establishing that they are benign cells. ■ The presence of cilia on a cell generally excludes a malignant diagnosis. ■ At times, bronchial cells can be large and have multiple nuclei, but the presence of cilia suggests that they are benign cells with reactive changes.1 10. A. Herpes simplex virus. Major points of discussion ■ After lung transplantation, patients are at risk for developing infections and lymphoma. ■ Immunosuppressed patients, such as those with HIV-AIDS, are also at risk for opportunistic infections. ■ An alveolar cast of P. carinii appears as a sheet of amorphous material. ■ The Gomori methenamine silver stain highlights pneumocystis and other fungal organisms, such as Aspergillus, Candida, and Mucor. ■ Candida organisms, associated with benign squamous cells in a BAL specimen, suggest that the specimen may represent oral contamination.1,3–5 11. A. Pneumothorax. Major points of discussion ■ Effusions can have lymphocytes, neutrophils, and/or eosinophils. ■ Eosinophils are frequently associated with parasitic infections, but their presence in a pleural effusion is most commonly linked to introduction of air into the pleural space. ■ The differential diagnosis for eosinophils in a pleural effusion includes infection and drug reaction. ■ Abundant granular debris in an effusion is associated with rheumatoid arthritis. ■ A hematoxylin body or LE cell may be seen in patients with lupus.1,3-5 12. A. Carcinoma. Major points of discussion ■ Microscopic description: A structure with an elongated, iron-coated, dumbbell shape and clear core. ■ An asbestos body is a subtype of a ferruginous body. ■ A clear core is suggestive of an asbestos body. ■ A non-asbestos ferruginous body has a black or brown core. ■ Identification of an asbestos body in a BAL specimen indicates that the person has had asbestos exposure. ■ The presence of an asbestos body does not signify that the patient has mesothelioma.1,3-5 13. A. Aspergillosis. Major points of discussion ■ The differential for budding yeast includes Candida sp., Histoplasma capsulatum, and C. neoformans. ■ Histoplasma organisms are typically intracellular, within macrophages. ■ The thickness of hyphae, septation, and angle of hyphal branching are useful in differentiating among Candida, Aspergillus, and Mucor. ■ A mucicarmine stain is helpful in confirming a diagnosis of Cryptococcus. ■ Occasionally strands of mucus and elastic fibers can mimic fungi. A silver stain is helpful in differentiating between these mimics and fungi.1,3-5 14. A. Negative; tuberculosis. Major points of discussion ■ Pleural effusions and ascites fluid typically have a combination of mesothelial cells and histiocytes. ■ Both mesothelial cells and histiocytes are normal constituents of the pleural and peritioneal cavities. ■ Benign mesothelial cells and histiocytes are present as single cells. In pelvic washing specimens, mesothelial cells shed as two-dimensional sheets. ■ Identification of clusters of epithelioid cells and two-celled populations suggest malignancy. Immunostains may be necessary to confirm the morphologic impression and determine the identity of the primary tumor. ■ Lymphocytes, whether benign or neoplastic, occur as single cells. Lymphocytes are smaller than mesothelial cells and histiocytes.1 15. A. Malignant; lymphoma. Major points of discussion ■ A signet ring cell has an intracytoplasmic vacuole that compresses the nucleus to one side. These cells are characteristic of adenocarcinomas. ■ In a malignant effusion, the presence of vacuolated cytoplasm is typical of adenocarcinoma. ■ The presence of psammoma bodies (spherical concentrically laminated collections of calcium) in a malignant effusion is characteristic of an adenocarcinoma. ■ Abundant extracellular mucin in a malignant effusion is consistent with adenocarcinoma. ■ Dense cytoplasm and windows between malignant cells are suggestive of mesothelioma. However, immunostains are typically performed to confirm the morphological impression.1 16. A. Uric acid crystals. Major points of discussion ■ Crystals formed from the same substance can have various shapes. ■ Abnormal crystals include cystine, tyrosine, leucine, and sulfadiazine. ■ Uric acid crystals and cystine crystals can be hexagonal. ■ Uric acid crystals, unlike some cystine crystals, are birefringent under polarized light. ■ Crystal formation in urine is dependent on pH, temperature, and concentration of the substance. 17. A. Chondrocytes. Major points of discussion ■ Chondrocytes have no pathologic significance. ■ Chondrocytes can be present as single cells or clusters. ■ Chondrocytes are obtained from inadvertent sampling of cartilage. ■ Numerous red blood cells, not chondrocytes, are suggestive of a traumatic tap. ■ Chondrocytes can be mistaken for adenocarcinoma, including one with signet ring cell features. 18. A. Toxoplasmosis. Major points of discussion ■ Toxoplasma tachyzoites are present both intracellularly and extracellularly. ■ Toxoplasma organisms (3 to 6 μm) are smaller than cryptococci, which range from 5 to 15 μm in diameter. ■ A capsule surrounds cryptococci. ■ Cryptococci demonstrate narrow-based budding. ■ The capsule of cryptococci stains with mucicarmine. There are also capsule-deficient forms of cryptococci. 19. A. Normal findings. Major points of discussion ■ Normal CSF has rare cells. ■ Small, round blue cell tumors include lymphoma/leukemia, small cell carcinoma, and medulloblastoma. ■ Nuclear molding may be present in medulloblastoma and small cell carcinoma. ■ Small cell carcinoma lacks nucleoli. ■ Small cell carcinoma is more likely to be seen in adults, whereas medulloblastoma affects children. 20. A. White blood cell cast; no clinical significance. Major points of discussion ■ Urinary casts are cylindrical structures formed in the kidney. ■ When dislodged, casts can be detected in urine specimens. ■ Casts can be associated with pathologic and nonpathologic conditions. ■ Casts can be composed of various cell types, including red blood cells, leukocytes, and renal tubular cells. ■ The width of a cast can vary. Broader casts arise from dilated tubules/ducts, whereas thinner ones form in compressed tubules. 21. A. White blood cell cast; no clinical significance. Major points of discussion ■ Urinary casts are cylindrical structures formed in the kidney. ■ When dislodged, casts can be detected in urine specimens. ■ Casts can be associated with pathologic and nonpathologic conditions. ■ Casts can be composed of various cell types, including red blood cells, leukocytes, and renal tubular cells. ■ The width of a cast can vary. Broader casts arise from dilated tubules/ducts, whereas thinner ones form in compressed tubules. 22. A. Uric acid crystals. Major points of discussion ■ Crystals formed from the same substance can have various shapes. ■ Abnormal crystals include cystine, tyrosine, leucine, and sulfadizine. ■ Uric acid crystals and cystine crystals can be hexagonal. ■ Uric acid crystals, unlike some cystine crystals, are birefringent under polarized light. ■ Crystal formation in urine is dependent on pH, temperature, and concentration of the substance. 23. A. White blood cell cast; no clinical significance. Major points of discussion ■ Urinary casts are cylindrical structures formed in the kidney. ■ When dislodged, casts can be detected in urine specimens. ■ Casts can be associated with pathologic and nonpathologic conditions. ■ Casts can be composed of various cell types, including red blood cells, leukocytes, and renal tubular cells. ■ The width of a cast can vary. Broader casts arise from dilated tubules/ducts, whereas thinner ones form in compressed tubules.
Body Fluids and Clinical Microscopy
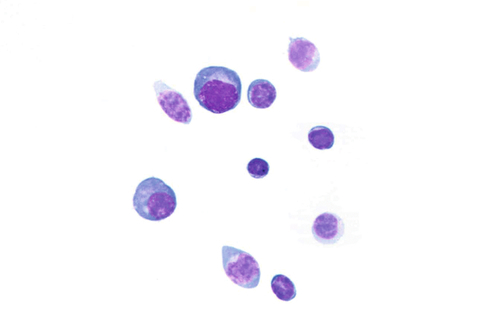
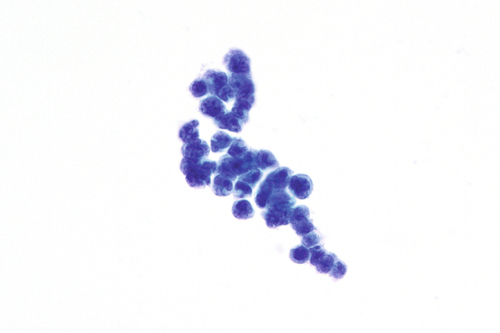
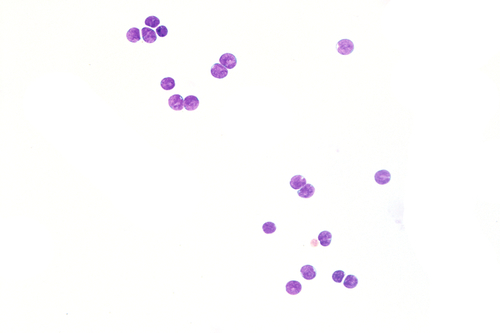
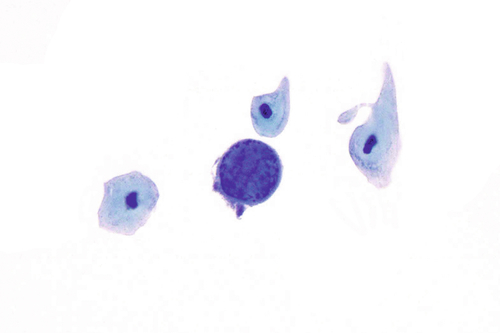
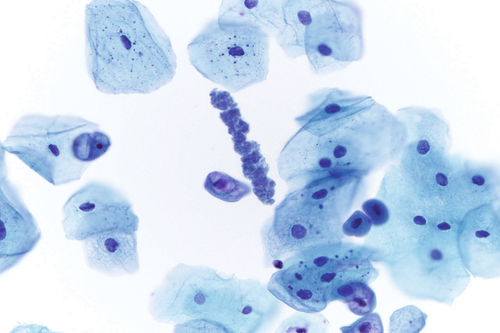
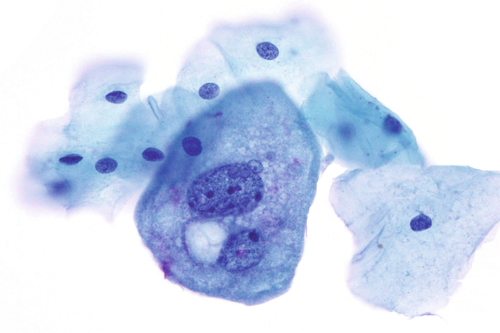
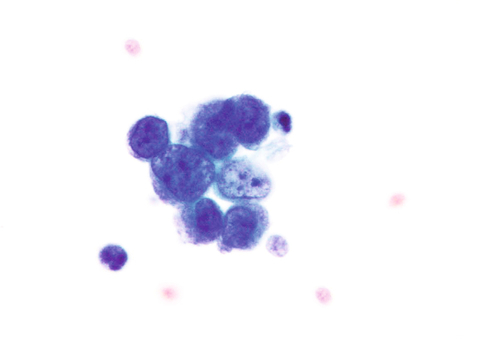

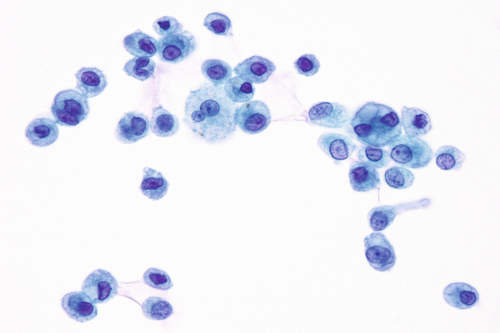
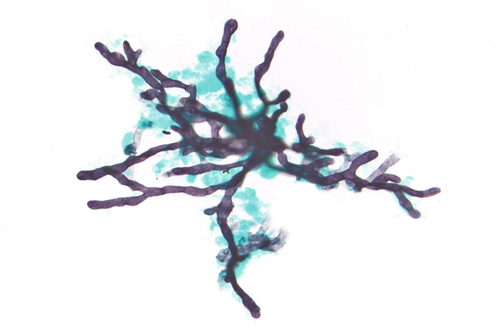
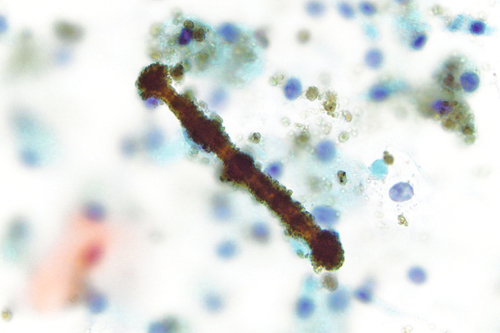
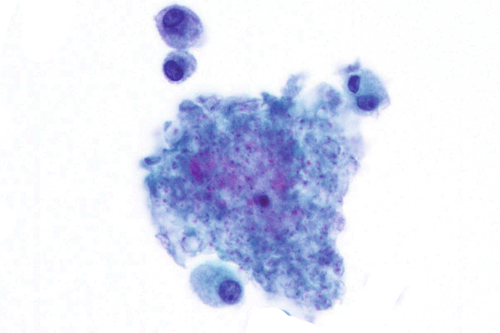
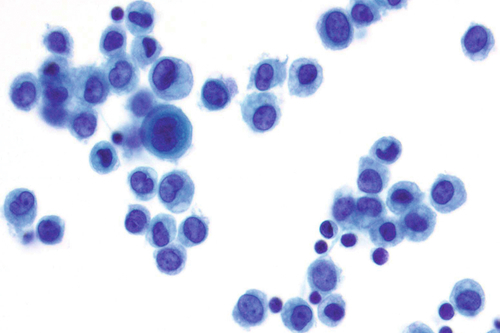
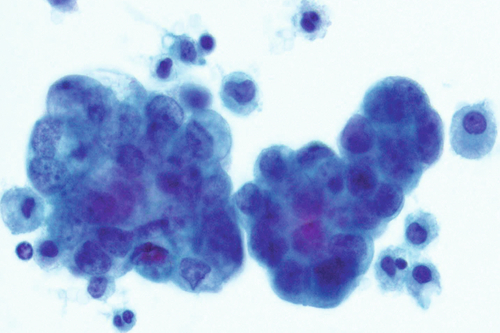
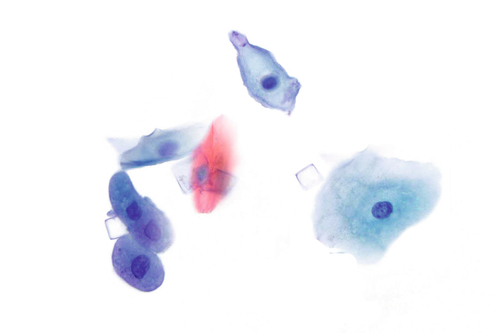

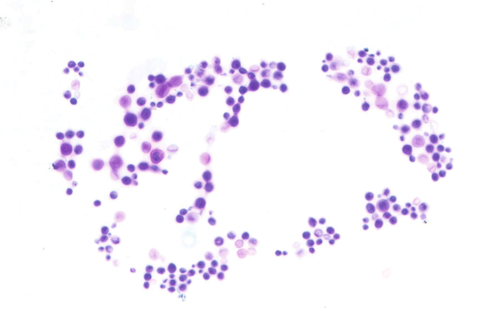
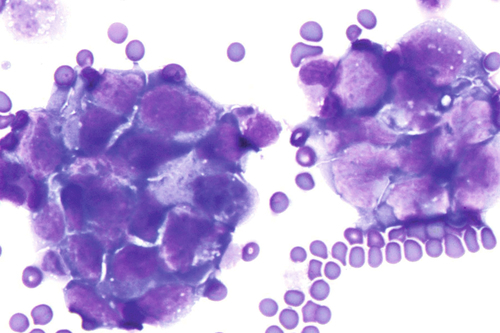
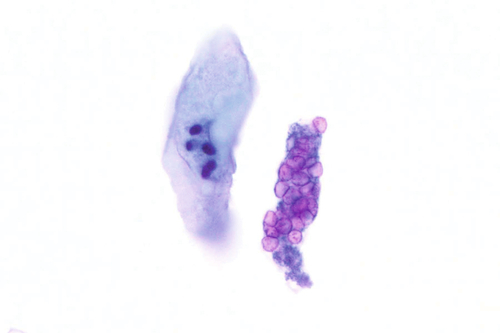
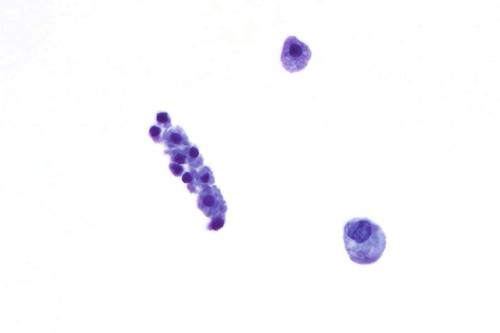
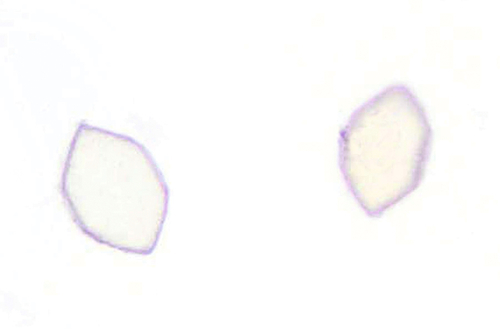
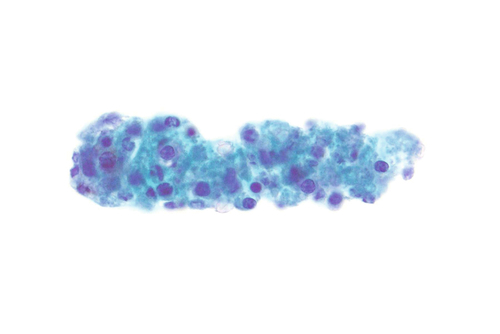
Rationale: A normal CSF is sparsely cellular and contains only a few small, mature-appearing lymphocytes and monocytoid cells, which have moderate cytoplasm and folded or kidney bean–shaped nuclei.
B. Bacterial meningitis.
Rationale: CSF from a patient with bacterial meningitis is likely to be rich in neutrophils. However, neutrophils accompanied by numerous red blood cells are suggestive of peripheral blood contamination. Toxoplasmosis meningoencephalitis and early stages of viral meningitis are also in the differential diagnosis for a CSF sample with increased neutrophils.
C. Aseptic meningitis.
Rationale: CSF, as seen in Figure 13-1, from a patient with aseptic meningitis is likely to have increased numbers of lymphocytes. Plasma cells and monocytoid cells may also be present. An infectious organism, usually a virus, often causes aseptic meningitis. However, bacteria, fungi, systemic diseases, and medications can also lead to aseptic meningitis.
D. Parasitic infection.
Rationale: The presence of numerous eosinophils, which is a rare finding in CSF, is suggestive of a parasitic infection, such as one caused by Taenia solium or Angiostrongylus cantonensis.
E. Lymphoma.
Rationale: Numerous lymphoid cells are present in the CSF from a patient with lymphoma. However, the cells are more monotonous than the polymorphous population illustrated in the image.
Rationale: Metastatic breast carcinoma is among the most commonly encountered malignancies in adult women. Other common malignancies seen in CSF samples from adults include lung carcinoma, melanoma, lymphoma, and leukemia. Breast carcinoma cells occur singly or in clusters and lack nuclear molding.
B. Metastatic lung small cell carcinoma.
Rationale: Small cell carcinoma, as seen in Figure 13-2, has cells with scant cytoplasm, nuclear molding, and no nucleoli. The lung is often a source of occult primary tumors presenting as brain metastases, as in this case. There is morphological overlap between small cell carcinoma and medulloblastoma, but the latter predominantly affects children.
C. Metastatic melanoma.
Rationale: Metastatic melanoma is also among the common tumors presenting in the CSF. Melanoma can take on many morphological forms, including pigmented or nonpigmented, epithelioid or spindled, single cells or clusters, and monotonous or pleomorphic. However, melanoma cells lack the nuclear molding seen in this figure.
D. Metastatic lymphoma.
Rationale: Malignant lymphoid cells tend to occur singly and not in clusters. Nuclear molding is not a feature of lymphomas. In addition, diffuse large B-cell lymphoma, a subtype of lymphoma, likely involves the CSF and has coarse chromatin, prominent nucleoli, and irregular nuclear contours.
E. Metastatic lung adenocarcinoma.
Rationale: Lung adenocarcinoma, like breast carcinoma, consists of atypical epithelial cells present singly and in clusters. Sometimes the clusters form rounded structures resembling glands, and the cells have intracytoplasmic mucin, suggestive of an adenocarcinoma.
Rationale: The CSF from a patient with aseptic meningitis typically has a polymorphous lymphoid population consisting of small, intermediate, and few large lymphocytes, some of which may appear atypical. Plasma cells and monocytoid cells can also be seen in the background.
B. Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Rationale: CLL/SLL is composed of small lymphocytes, which are round to slightly irregular. CLL/SLL is a neoplasm of adulthood. Lymphomas, although they can have a morphologic appearance similar to that seen in Figure 13-3, tend to affect adults. Therefore, the clinical history, especially the age of the patient, is critical in formulating a differential diagnosis and arriving at the correct diagnosis.
C. Acute lymphoblastic leukemia (ALL).
Rationale: ALL arises in the bone marrow and most commonly affects children between the ages of 2 and 7 years. Lymphoblasts vary in size from small blasts with scant cytoplasm to larger cells with moderate cytoplasm. The age and clinical history are useful in interpreting the CSF in this setting. A diagnosis of ALL is usually established first, and then a lumbar puncture is performed to evaluate involvement of the central nervous system.
D. Medulloblastoma.
Rationale: Medulloblastoma, a small, round blue cell tumor of childhood, is also in the differential diagnosis in this case. Nuclear molding in medullablastoma can aid in distinguishing it from ALL and other lymphoid entities, which occur as single, noncohesive cells. Nucleoli in medulloblastoma may be inconspicuous or prominent.
E. Bacterial meningitis.
Rationale: The CSF from a patient with bacterial meningitis will predominantly have neutrophils, rather than lymphoid cells, as seen in this case.
Rationale: Urothelial carcinoma in a voided urine specimen presents as atypical urothelial cells present singly and/or in clusters. Atypical features include a high nuclear/cytoplasmic ratio, hyperchromasia, coarse chromatin, and irregular nuclear borders. At times, large nucleoli may be present.
B. Positive for malignant cells; renal clear cell (conventional) carcinoma.
Rationale: Renal clear cell (conventional) carcinoma is the most common renal cell carcinoma, but is infrequently seen in voided urine specimens. Tumor cells have abundant cytoplasm that is vacuolated and round to slightly irregular nuclei. Nucleoli may be inconspicuous or prominent, depending on the grade of the tumor.
C. Negative for malignant cells; polyomavirus infection.
Rationale: Urothelial cells infected by polyomavirus (BK virus), as in this case, can mimic carcinoma because of the increased nuclear size and hyperchromasia; therefore, they are sometimes referred to as “decoy cells.” The cells occur singly and the nuclei are round, homogeneous, and smudgy. Although human polyomavirus can affect immunocompromised patients, it can also be found in urine specimens of patients with no predisposing conditions.
D. Negative for malignant cells; hyaline inclusion bodies.
Rationale: Hyaline inclusion bodies are eosinophilic cytoplasmic inclusions of varying sizes frequently seen in degenerating urothelial cells.
E. Negative for malignant cells; renal tubular cells.
Rationale: Renal tubular cells are small cells, approximately the size of lymphocytes, with granular cytoplasm and dark nuclei.
Rationale: All casts appear as long tubular structures. Granular casts, as represented in Figure 13-5, are physiologic and may be present in normal urine specimens. Increased numbers of granular casts may be seen following physical stress. Other types of casts, including red blood cell casts, white blood cell casts, fatty casts, and degenerated renal tubular casts, are associated with underlying renal disease.
B. Hyaline inclusion body.
Rationale: Hyaline inclusion bodies, also known as globular inclusions or Melamed-Wolinska bodies, are round, red, eosinophilic, cytoplasmic structures of varying sizes thought to represent lysosomes. They are often present in degenerated urothelial cells. Most importantly, they do not represent viral inclusions.
C. Corpora amylacea.
Rationale: Corpora amylacea are round structures with concentric laminations derived from prostatic secretions.
D. Trichomoniasis.
Rationale: In urine specimens, trichomoniasis usually represents contamination from the female genital tract, but it may also be a source of prostatitis. The Trichomonas organism is pear shaped, with flagella that are sometimes visible.
E. Calcium oxalate crystal.
Rationale: Crystals are commonly seen in urine specimens and most are clinically insignificant. They can be of various shapes and sizes. Calcium oxalate crystals typically resemble an envelope, although they can take on other morphologic forms.
Rationale: Endometrial cells are sometimes found incidentally in urine specimens obtained from women. Endometrial cells form tight three-dimensional clusters, and each cell is approximately the size of a lymphocyte and has a slightly irregular nucleus.
B. Squamous cell.
Rationale: Squamous cells can be similar in size to umbrella cells. Unlike umbrella cells, normal squamous cells have more homogenous and less grainy cytoplasm. When present in large numbers in voided urine specimens obtained from women, squamous cells likely represent vaginal contamination.
C. Renal tubular cell.
Rationale: Renal tubular cells are small cells, approximately the size of lymphocytes. Unlike umbrella cells, they have scant cytoplasm and a dark-staining nucleus.
D. Basal cell.
Rationale: Basal (i.e., transitional) cells also have slightly granular cytoplasm, like umbrella cells. However, they are intermediate in size; they are smaller than umbrella cells, but larger than renal tubular cells.
E. Umbrella cell.
Rationale: An umbrella cell, as seen in Figure13-6, is a normal finding in urine specimens. They are larger than all other urothelial cells and can have one or multiple nuclei. The low nuclear/cytoplasmic ratio distinguishes them from malignant cells. Histologically, umbrella cells cap the basal (i.e., transitional) cells.
Rationale: In a normal voided urine specimen, urothelial cells occur singly. Although umbrella cells are large and can have multiple nuclei, they have low nuclear/cytoplasmic ratios and lack hyperchromasia, compatible with a benign process.
B. Positive for malignant cells; urothelial carcinoma.
Rationale: Cells from urothelial carcinomas can present singly and/or in clusters. High nuclear/cytoplasmic ratios, hyperchromasia, coarse chromatin, and nuclear irregularities, as seen in Figure 13-7, distinguish malignant cells from benign urothelial cells.
C. Negative for malignant cells; instrumentation effect.
Rationale: Benign transitional cells may occur as clusters if they are obtained during/following instrumentation or catheterization. In these scenarios, the “clusters” often consist of two-dimensional sheets and the cells lack atypical features, such as high nuclear/cytoplasmic ratios, hyperchromasia, coarse chromatin, and nuclear irregularities.
D. Positive for malignant cells; lymphoma.
Rationale: In a cytology preparation, lymphoid cells, whether in urine or body fluid from another site, occur singly. Cell clusters are suggestive of an epithelioid origin.
E. Negative for malignant cells; vaginal contamination.
Rationale: Urine specimens from women frequently have an abundance of benign squamous cells that likely originated in the vagina. However, squamous cells do not always represent vaginal contamination in women and are also present in urine specimens obtained from men. In these scenarios, the squamous cells likely originate from metaplasia in the trigone of the bladder or urethra. Benign squamous cells occur singly and have low nuclear/cytoplasmic ratios.
Rationale: Most crystals have no clinical relevance. Crystals are commonly found in urine specimens and their presence in urine is related to pH, concentration, and temperature. Calcium oxalate crystals are not pathologic. Typically, they are square to rectangular in shape with a cross resembling the backside of an envelope, but they may also take other forms and be ovoid or dumbbell-shaped.
B. Triple phosphate crystals; nonpathologic.
Rationale: Triple phosphate crystals are rectangular in shape and bear resemblance to coffin lids; they are not clinically significant.
C. Uric acid crystals; pathologic.
Rationale: Uric acid crystals are not pathologic. They can have different shapes, including flat plates and pointed ovals.
D. Cystine crystals; nonpathologic.
Rationale: The presence of cystine crystals may signify cystinuria or cystine calculi. Cystine crystals are hexagonal and laminated.
E. Tyrosine crystals; nonpathologic.
Rationale: Tyrosine crystals may be seen in patients with liver disease. They look like thin needles in clusters.
Rationale: Adenocarcinoma in the lung presents as cell clusters and usually with single cells. The cytoplasm of the cells can be vacuolated like macrophages, but the atypia, consisting of high nuclear/cytoplasmic ratios, hyperchromasia, and nuclear irregularities, distinguishes adenocarcinoma from benign cells. Poorly differentiated carcinomas may have all of the above listed atypical features, whereas the atypia in well-differentiated adenocarcinomas may be focal and subtle.
B. Positive for malignant cells;lymphoma.
Rationale: Following transplantation, patients are at risk for developing lymphoma. Lymphoid cells occur singly but are smaller and have scant cytoplasm compared with macrophages.
C. Negative for malignant cells; benign bronchial cells.
Rationale: Occasional bronchial cells are present in BAL specimens. Depending on their orientation, bronchial cells may appear as columnar cells with terminal bars and cilia, or as two-dimensional honeycombed sheets. Monotony and blandness of the cells are useful in recognizing that they are benign.
D. Negative for malignant cells; pulmonary alveolar macrophages.
Rationale: Numerous pulmonary alveolar macrophages are normally seen in BAL specimens. They occur singly, have low nuclear/cytoplasmic ratios, vacuolated cytoplasm, round to ovoid nuclei, and no hyperchromasia.
E. Negative for malignant cells; benign squamous cells.
Rationale: Benign squamous cells, often representing oral/upper airway contamination, are occasionally encountered in BALs. Unlike pulmonary alveolar macrophages, which are vacuolated, squamous cells have a dense, homogeneous cytoplasm.
Rationale: Cells infected with herpes tend to be multinucleated and have nuclear molding and smudgy chromatin.
B. Candida sp.
Rationale: Candida may appear as budding yeast or pseudohyphae.
C. Aspergillus sp.
Rationale: Aspergillus is a fungus with hyphae that show septation and acute 45-degree branching.
D. Cytomegalovirus.
Rationale: Cells infected with cytomegalovirus have intranuclear inclusions surrounded by a halo.
E. Pneumocystis carinii.
Rationale: In a BAL, P. carinii can be identified by the presence of foamy alveolar casts. A silver stain can be performed to confirm the diagnosis.
Rationale: Eosinophilic effusions are most commonly caused by prior sampling (e.g., placement of a chest tube for iatrogenic pneumothorax caused by a transthoracic lung biopsy) and the introduction of air or blood into the pleural space.
B. Drug reaction.
Rationale: A drug reaction can lead to eosinophils in an effusion, but it is a less common cause of an eosinophilic effusion.
C. Parasitic infection.
Rationale: A parasitic infection can lead to eosinophilia in an effusion, but it is a less common cause of an eosinophilic effusion.
D. Rheumatoid arthritis.
Rationale: A pleural effusion from a patient with rheumatoid arthritis is typically rich in macrophages and has abundant granular debris. Occasional lymphocytes and neutrophils may be seen. Eosinophils are not typically associated with rheumatoid pleuritis.
E. Systemic lupus erythematosus.
Rationale: An effusion from a patient with lupus pleuritis may have characteristic lupus erythematosus (LE) cells, which consist of neutrophils or macrophages with eccentrically-located nuclei and ingested degenerated material. This material, called a hematoxylin body, has a glassy, homogeneous appearance. Eosinophils are not associated with systemic lupus erythematous.
Rationale: Figure 13-11 shows predominantly pulmonary macrophages, which is a normal finding in BAL specimens. Pulmonary macrophages have low nuclear/cytoplasmic ratios, nuclei with fine chromatin, and abundant vacuolated cytoplasm. Most carcinomas will form at least some clusters, have cells with high nuclear/cytoplasmic ratios, and contain nuclei with irregular contours and coarse chromatin.
B. Mesothelioma.
Rationale: Mesothelioma usually presents in a pleural effusion (and not in a BAL specimen) as cell clusters and single cells. In contrast, macrophages tend to occur singly. Also, the presence of an asbestos body signifies asbestos exposure, but it is not a sine qua non of mesothelioma.
C. Pulmonary hemorrhage.
Rationale: A patient with pulmonary hemorrhage will have hemosiderin-laden macrophages, characterized by golden-brown, intracytoplasmic particles of varying sizes. No hemosiderin is seen in the macrophages in this figure.
D. Aspiration pneumonia.
Rationale: The presence of food particles and inflammation in a BAL specimen is highly suggestive of aspiration.
E. Asbestos exposure.
Rationale: The figure demonstrates an elongated, dumbbell-shaped structure with a clear core coated by iron; this is consistent with an asbestos body. Patients with asbestos exposure may have asbestos bodies in the lung parenchyma and pleural plaques (thickening), as described in this patient. An asbestos body is a form of a ferruginous body. Non-asbestos ferruginous bodies have black or brown cores, in contrast to the clear cores seen in asbestos bodies.
Rationale: Aspergillus sp. pneumonia tends to affect immunosuppressed individuals. Aspergillus is a fungal organism that is characterized by septated hyphae with acute, 45-degree angle branching.
B. Mucormycosis.
Rationale: Mucormycosis is typically seen in patients with underlying hematopoietic malignancies or diabetes mellitus. Relative to Aspergillus, Mucor organisms have broader hyphae that are nonseptated and have irregular branching.
C. Histoplasmosis.
Rationale: Histoplasmosis may be asymptomatic or symptomatic, especially in immunosuppressed patients. The organisms are often intracellular, within macrophages, and consist of narrow-based, round to ovoid-shaped, budding yeast.
D. Pneumocystis infection.
Rationale: Immunosuppressed patients, such as those with HIV-AIDS, are susceptible to P. carinii infection. Vacuolated foamy casts are diagnostic of the organisms. A silver stain highlights the cysts.
E. Cryptococcosis.
Rationale: Immunocompetent and immunosuppressed patients are at risk for developing infections with Cryptococcus neoformans. Microscopically, C. neoformans consists of narrow-budding yeast surrounded by a mucinous capsule.
Rationale: An effusion from a patient with tuberculosis is composed predominantly of lymphocytes and rare mesothelial cells. A similar pattern may be observed in effusions from patients with lymphoma/leukemia, recent cardiac surgery, or sarcoidosis. Clinical history and ancillary tests are helpful is determining the etiology of the lymphocytes.
B. Malignant; adenocarcinoma.
Rationale: Carcinomas in fluids will occur as clusters and single cells. A second cell population that does not resemble mesothelial cells or histiocytes can be helpful in identifying adenocarcinoma. Features helpful in making a diagnosis of malignancy include cells with high nuclear/cytoplasmic ratios, prominent nucleoli, enlarged cells, and irregular nuclear contours. Poorly differentiated carcinomas may have all of these features, whereas well-differentiated carcinomas may lack some of these characteristics.
C. Negative; mostly histiocytes.
Rationale: Most benign effusions are composed of mesothelial cells and histiocytes, which tend to occur as single cells. In contrast to mesothelial cells, histiocytes have more vacuolated cytoplasm, folded or bean-shaped nuclei, and no “windows.”
D. Malignant; lymphoma.
Rationale: Lymphomas present as single cells, and the type of lymphoma dictates other cytologic features. For example, an effusion caused by a diffuse large B-cell lymphoma has large lymphoid cells with prominent nucleoli. In contrast, one caused by follicular lymphoma is composed of relatively smaller cells with cleaved nuclei.
E. Negative; mostly mesothelial cells.
Rationale: Mesothelial cells tend to have dense cytoplasm. When they do aggregate, mesothelial cells have a small space or window between them resulting from intervening long microvilli.
Rationale: Lymphomas present predominantly as single cells. The morphology varies with the type of lymphoma, ranging from small cells to large cells with prominent nucleoli.
B. Benign; histiocytes.
Rationale: Histiocytes are present singly, have foamy cytoplasm, and low nuclear/cytoplasmic ratios.
C. Malignant; mesothelioma.
Rationale: Because the cells in both mesotheliomas and adenocarcinomas form cell clusters, distinguishing between them can be challenging and immunostains are often required for definitive diagnosis. Morphologic features in favor of mesothelioma include the presence of windows and dense cytoplasm. Cytoplasmic vacuolization and signet ring cells support a diagnosis of adenocarcinoma.
D. Benign; mesothelial cells.
Rationale: In effusions, benign mesothelial cells tend to occur singly. If they aggregate, mesothelial cells have a space or window between them. In addition, they have round nuclei and dense cytoplasm.
E. Malignant; adenocarcinoma.
Rationale: Figure 13-14 shows two populations of cells. There are smaller cells that are singly dispersed and represent an admixture of benign mesothelial cells and histiocytes. The second population is composed of larger atypical cells present mostly as cohesive clusters, consistent with adenocarcinoma. The presence of vacuolated cytoplasm aids in rendering a diagnosis of adenocarcinoma and in distinguishing these cell clusters from those of mesothelioma.
Rationale: Uric acid crystals can have various shapes. Some uric acid crystals are hexagonal. In contrast to cystine crystals, uric acid crystals are birefringent under polarized light. Increased numbers of uric acid crystals may be associated with increased urates in patients undergoing therapy for leukemia/lymphoma.
B. Cystine crystals.
Rationale: Cystine crystals, like some uric acid crystals, are hexagonal with equal or unequal sides. Unlike uric acid crystals, thin forms of cystine crystals do not polarize. Cystinuria, an autosomal recessive disease, may lead to the development of calculi in the urinary tract.
C. Tyrosine crystals.
Rationale: Tyrosine crystals occur as groups of fine needles and may coexist with leucine crystals, which are spherical. Tyrosine crystals are present in patients with underlying liver disease who are unable to metabolize the amino acid.
D. Calcium oxalate crystals.
Rationale: The image shows urothelial cells, the two cells in the lower left with more opaque cytoplasm; and squamous cells, which have more transparent cytoplasm. Interspersed are square crystals. (Diagonal lines are not illustrated here.) Calcium oxalate crystals are colorless squares with intersecting diagonal lines resembling an envelope. Dumbbell-shaped and ovoid forms also occur. Large numbers of calcium oxalate crystals may suggest the presence of chronic renal disease or toxicity from ethylene glycol or methoxyflurane.
E. Sulfadiazine crystals.
Rationale: Drugs excreted in the urine may form crystals. The configuration of the sulfadiazine crystals depends on the specific drug. One form appears as wheat sheaves.
Rationale: Cartilage-containing chondrocytes, as seen in Figure 13-16, are sometimes present in CSF specimens. The cells can be present singly or in clusters. The combination of chondrocyte lacunae, which create paranuclear clearing, and large size can sometimes be mistaken for neoplastic epithelial cells. The slide shows chondrocytes with surrounding clear lacunae in cartilage.
B. Traumatic tap.
Rationale: A traumatic tap is composed of numerous red blood cells from peripheral blood contamination. Presence of cartilage is not an indication of a traumatic tap.
C. Glioblastoma multiforme (GBM).
Rationale: Cells from a GBM are large and have highly pleomorphic nuclei with coarse chromatin.
D. Signet ring adenocarcinoma.
Rationale: Cells from a signet ring cell carcinoma can be present singly or as clusters. In contrast to chondrocytes, adenocarcinoma has hyperchromatic and angulated nuclei, but sometimes the cells can appear bland. Signet ring cells contain mucin, which usually displaces the nucleus to the periphery. The nuclei of chondrocytes tend to be centrally located.
E. Old hemorrhage.
Rationale: A CSF with old hemorrhage will have hemosiderin-laden macrophages, which consist of macrophages with intracellular refractile, golden-brown pigment.
Rationale: Toxoplasma gondii causes meningoencephalitis in immunocompromised hosts. The crescent-shaped organisms are present either intracellularly or extracellularly. The background shows a mixture of neutrophils and mononuclear cells.
B. Cryptococcus.
Rationale: C. neoformans, as seen in Figure 13-17, is a fungus that can be seen in the CSF of both healthy and immunocompromised hosts. The yeast vary in size, have narrow-based budding, and the capsule is mucin positive. The amount of inflammation in the background can be variable.
C. Chondrocytes.
Rationale: Rarely, contamination by chondrocytes may be seen in CSF specimens as a result of inadvertent sampling of the vertebral column. In contrast to Cryptococci, chondrocytes are much larger and are surrounded by a thick matrix. The large size of chondrocytes may also be mistaken for a malignant neoplasm.
D. Contamination (starch).
Rationale: Starch, typically a contaminant from glove powder, can be mistaken for Cryptococcus. Unlike Cryptococcus, starch displays a Maltese cross under polarized light in the center and has no capsule or budding forms.
E. Parasitic infection.
Rationale: Parasitic infections are accompanied by numerous eosinophils. The structures in this figure represent fungi, and no significant inflammatory component is present.
Rationale: Normal CSF is typically sparsely cellular with rare, mature-appearing lymphocytes and monocytoid cells. These usually occur as singly dispersed cells.
B. Bacterial meningitis.
Rationale: Bacterial meningitis in CSF contains mostly neutrophils. Similar to lymphocytes and monocytoid cells, neutrophils occur as singly dispersed cells.
C. Aseptic meningitis.
Rationale: In contrast to a CSF with normal findings, a specimen from a patient with aseptic meningitis is more cellular and has a mixture of lymphocytes, plasma cells, and monocytoid cells.
D. Medulloblastoma.
Rationale: Medulloblastoma, as seen in this figure, is a small, round blue cell tumor that occurs primarily in children. Identification of nuclear molding is useful in distinguishing medulloblastoma from lymphoma/leukemia, another small, round blue cell tumor. Metastatic small cell carcinoma also demonstrates nuclear molding, but it affects primarily adults.
E. Small cell carcinoma.
Rationale: Small cell carcinoma has cells with scant cytoplasm, nuclear molding, and no nucleoli. The lung is often a source of occult primary tumors presenting as brain metastases. There is morphological overlap between small cell carcinoma and medulloblastoma, but the latter predominantly affects children.
Rationale: White blood cell casts are elongated structures with variable numbers of white blood cells.
B. White blood cell cast; clinically significant.
Rationale: These casts are clinically relevant and seen in patients who have tubulointerstitial disease and renal transplant rejection.
C. Red blood cell cast; no clinical significance.
Rationale: Red blood cell casts are associated with glomerulonephritis.
D. Red blood cell cast; clinically significant.
Rationale: Figure 13-9 demonstrates an epithelial cell adjacent to a tubular structure composed of numerous red blood cells, consistent with a red blood cell cast. Red blood cells casts are elongated structures containing outlines of red blood cells and are a sign of bleeding within nephrons.
E. Renal tubular epithelial cell casts; clinically significant.
Rationale: Renal tubular casts can resemble white blood cell casts. Identification of cells with relatively abundant granular cytoplasm is characteristic of renal tubular epithelial casts. Several disease states, including tubular necrosis, viral disease, and drug toxicity, are associated with renal tubular casts.
Rationale: White blood cell casts are elongated structures with variable numbers of white blood cells.
B. White blood cell cast; clinically significant.
Rationale: These casts are clinically relevant and seen in patients who have tubulointerstitial disease and renal transplant rejection.
C. Red blood cell cast; no clinical significance.
Rationale: Red blood cell casts are associated with renal disease.
D. Red blood cell cast; clinically significant.
Rationale: Red blood cells casts are elongated structures containing outlines of red blood cells and are a sign of bleeding within nephrons.
E. Renal tubular epithelial cell cast; clinically significant.
Rationale: The image shows two larger urothelial cells and a tubular structure composed of renal tubular cells, consistent with a renal tubular cast. Moderate and granular cytoplasm typical of renal tubular cells is helpful in distinguishing renal tubular cell casts from white blood cell casts. Renal tubular casts, as seen in Figure 13-20, can resemble white blood cell casts. Identification of cells with relatively abundant granular cytoplasm is characteristic of renal tubular epithelial casts. Several disease states, including tubular necrosis, viral disease, and drug toxicity, are associated with renal tubular casts.
Rationale: The image shows two hexagonal crystals compatible with uric acid crystals. Uric acid crystals can have various shapes. Some uric acid crystals are hexagonal, as seen in Figure 13-21. In contrast to cystine crystals, uric acid crystals are birefringent under polarized light. Numerous uric acid crystals may be associated with increased urates in patients undergoing therapy for leukemia/lymphoma.
B. Cystine crystals.
Rationale: Cystine crystals, like some uric acid crystals, are hexagonal with equal or unequal sides. Unlike uric acid crystals, thin forms of cystine crystals do not polarize. Cystinuria, an autosomal recessive disease, may lead to the development of calculi in the urinary tract.
C. Tyrosine crystals.
Rationale: Tyrosine crystals occur as groups of fine needles and may coexist with leucine crystals, which are spherical. Tyrosine crystals are present in patients with underlying liver disease who are unable to metabolize the amino acid.
D. Calcium oxalate crystals.
Rationale: Calcium oxalate crystals are colorless squares with intersecting diagonal lines resembling an envelope. Dumbbell-shaped and ovoid forms also occur. Large numbers of calcium oxalate crystals may suggest the presence of chronic renal disease or toxicity from ethylene glycol or methoxyflurane.
E. Sulfadiazine crystals.
Rationale: Drugs excreted in the urine may form crystals. The configuration of the sulfadiazine crystals depends on the specific drug. One form appears as wheat sheaves.
Rationale: White blood cell casts are elongated structures with variable numbers of white blood cells.
B. White blood cell cast; clinically significant.
Rationale: The image demonstrates a tubular structure with interspersed lymphocytes, consistent with a white blood cell cast. White blood cell casts, as seen in Figure 13-22, are clinically relevant and seen in patients who have tubulointerstitial disease and renal transplant rejection.
C. Red blood cell cast; no clinical significance.
Rationale: Red blood cell casts are associated with renal disease.
D. Red blood cell cast; clinically significant.
Rationale: Red blood cells casts are elongated structures containing outlines of red blood cells and are a sign of bleeding within nephrons.
E. Renal tubular epithelial cell casts; clinically significant.
Rationale: Renal tubular casts can resemble white blood cell casts. Identification of cells with relatively abundant granular cytoplasm is characteristic of renal tubular epithelial casts. Several disease states, including tubular necrosis, viral disease, and drug toxicity, are associated with renal tubular casts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


