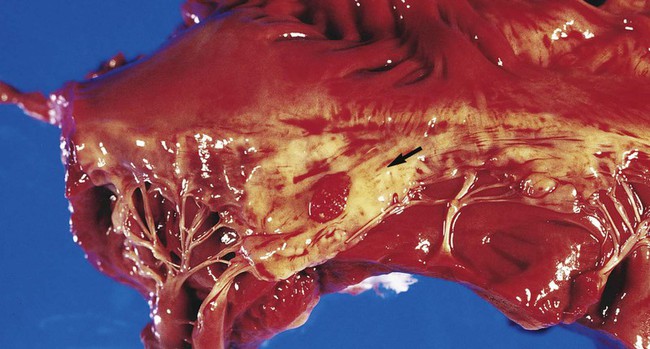
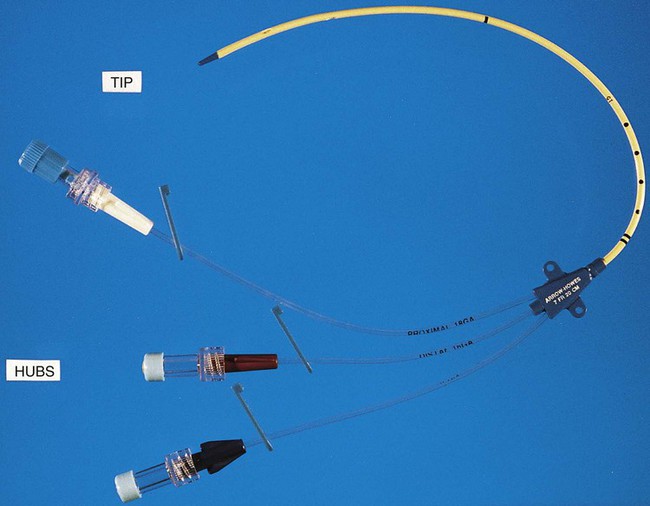
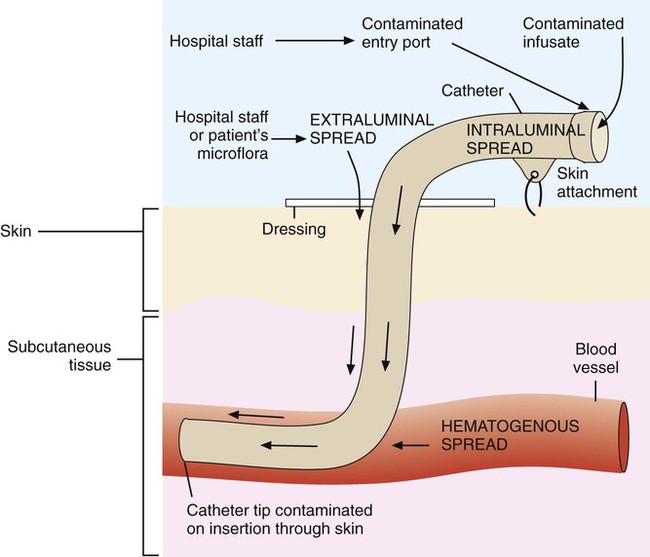
1. Identify and describe some of the medical consequences that occur when the bloodstream is infected by microorganisms. 2. Name the most common causes of bacterial bloodstream infection, and explain the route of transmission and source of infection. 3. Define the following bloodstream infections: bacteremia, fungemia, and septicemia. 4. List the most common fungi associated with bloodstream infections and the population of patients most often affected by this type of infection. 5. Explain what causes mortality in most cases of parasitic blood-borne infections. 6. Differentiate between intravascular and extravascular bloodstream infections. 7. Define continuous bacteremia, and provide an example. 8. Describe the development of infective endocarditis, including the contributing factors and the microorganisms that are the primary cause for the condition. 9. Define mycotic aneurysms and suppurative thrombophlebitis, and describe the causes for these conditions. 10. Explain the pathogenic features of S. epidermidis that make it uniquely suited for causing catheter-related infections. 11. Explain the importance of collection parameters associated with blood cultures for suspected cases of bloodstream infections, including collection time, the number of cultures, and the volume of blood required. 12. List and briefly describe some of the blood culture systems available to the microbiologist, including the self-contained systems, the lysis centrifugation systems, and instrument-based systems. 13. List some of the most common causes of bloodstream infection associated with the blood cultures from HIV-infected patients. 14. Define the acronym AACEK, and describe the type of blood-borne infections these organisms are most often associated with. 15. Outline the guidelines used to determine if agents isolated from blood cultures are true pathogens or probable contaminants. The organisms most commonly isolated from blood are gram-positive cocci, including coagulase-negative staphylococci, Staphylococcus aureus, and Enterococcus spp., and other organisms likely to be inhabitants of the hospital environment that colonize the skin, oropharynx, and gastrointestinal tract of patients. Some of the most common, clinically significant bacteria isolated from blood cultures are listed in Box 68-1. In general, the number of fungi and coagulase-negative staphylococci has increased, whereas the number of clinically significant anaerobic isolates has decreased since the early 2000s. Parasites in the bloodstream are usually detected by direct visualization. Those parasites for which traditional diagnosis is dependent on observation of the organism in peripheral blood smears include Plasmodium, Trypanosoma, and Babesia. Patients with malaria or filariasis may display a periodicity in their episodes of fever that allows the physician to time the collection of blood for microscopic examination intended for optimal detection. Rapid serological methods and molecular methods are currently used to detect malaria, babesiosis, and trypanosomiasis. These tests are described in Chapter 49. The development of infective endocarditis (infection of the endocardium most commonly caused by bacteria) is believed to involve several independent events. Cardiac abnormalities, such as congenital valvular diseases that lead to turbulence in blood flow or direct trauma from IV catheters, can damage cardiac endothelium. This damage to the endothelial surface results in the deposition of platelets and fibrin. If bacteria transiently gain access to the bloodstream (this can occur after an innocuous procedure such as brushing the teeth) after alteration of the capillary endothelial cells, the organisms may stick to and then colonize the damaged cardiac endothelial cell surface. After colonization, the surface will rapidly be covered with a protective layer of fibrin and platelets. This protective environment is favorable to further bacterial multiplication. This web of platelets, fibrin, inflammatory cells, and entrapped organisms is called a vegetation (Figure 68-1). The resulting vegetations ultimately seed bacteria into the blood at a slow but constant rate. The primary causes of infective endocarditis are the viridans streptococci, comprising several species (Box 68-2). These organisms are normal inhabitants of the oral cavity, often gaining entrance to the bloodstream as a result of gingivitis, periodontitis, or dental manipulation. Heart valves, especially those previously damaged, present convenient surfaces for attachment of these bacteria. Streptococcus sanguis and Streptococcus mutans are frequently isolated in streptococcal endocarditis. Gram-negative bacilli, known as the AACEK group, Aggregatibacter aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae, can also be associated with endocarditis. IV catheters are an integral part of the care for many hospitalized patients. More than 3 million central venous catheters are used annually in the United States. For example, central venous catheters are used to administer fluids, blood products, medications, antibiotics, and nutrition, and for hemodynamic monitoring. A short-term, triple-lumen (channel opening within a tube) central venous catheter is shown in Figure 68-2. Unfortunately, a major consequence of these medical devices is colonization of the catheter by either bacteria or fungi, which can lead to catheter infection and serious bloodstream infection. This consequence is a major nosocomial source of illness and even death. IV catheter–associated bacteremia (or fungemia) is believed to occur primarily by two routes (Figure 68-3). The first route involves the movement of organisms from the catheter entry site through the patient’s skin and down the external surface of the catheter to the catheter tip within the bloodstream. After arriving at the tip, the organisms multiply and may cause a bacteremia. The second way that IV catheter–associated bacteremia may occur is by migration of organisms along the inside of the catheter (the lumen) to the catheter tip. The catheter’s hub, where tubing connects into the IV catheter, is considered the site at which organisms gain access to the patient’s bloodstream through the catheter lumen. The most common etiologic agents for IV catheter–associated bloodstream infections, regardless of the route of infection, are organisms found on the skin (Box 68-3). Certain strains of S. epidermidis appear to be uniquely suited for causing catheter-related infections because of their ability to produce a biofilm or “slime” that consists of complex sugars (polysaccharides) believed to help the organism adhere to the catheter’s surface. The initial attachment of S. epidermidis to the catheter’s polystyrene surface is related to a cell surface protein. Once attached, the organism proliferates, subsequently forming a biofilm. Uncommon routes of IV catheter–tip infection include contaminated fluids or blood-borne seeding from another infection site. The most common portals of entry for bacteremia are the genitourinary tract (25%), respiratory tract (20%), abscesses (10%), surgical wound infections (5%), biliary tract (5%), miscellaneous sites (10%), and uncertain sites (25%). For the most part, the probability of bacteremia occurring from an extravascular site depends on the site of infection, its severity, and the organism. For example, any organism producing meningitis is likely to produce bacteremia at the same time. Of importance, certain organisms causing extravascular infections commonly invade the bloodstream; some of these organisms are listed in Table 68-1. In addition to these organisms, a large number of other bacteria and fungi that cause extravascular infections are also capable of invading the bloodstream. Whether these organisms invade the bloodstream depends on the host’s ability to control the infection and the organism’s pathogenic potential. Some of the organisms associated with potential bloodstream infections from a localized site include members of the family Enterobacteriaceae, Streptococcus pneumoniae, Staphylococcus aureus, Neisseria gonorrhoeae, anaerobic cocci, Bacteroides, Clostridium, beta-hemolytic streptococci, and Pseudomonas. These are only some of the organisms frequently isolated from blood. Almost every known bacterial species and many fungal species have been implicated in extravascular bloodstream infections. TABLE 68-1 Organisms Commonly Associated with Bloodstream Invasion from Extravascular Sites of Infection Shock is the gravest complication of septicemia. In septic shock, the presence of bacterial products and the host’s response act to shut down major host physiologic systems. Clinical manifestations include a drop in blood pressure, increase in heart rate, functional impairment in vital organs (brain, kidney, liver, and lungs), acid-base alterations, and bleeding problems. Gram-negative bacteria contain a substance in their cell walls, called endotoxin, which has a strong effect on several physiologic functions. This substance, a lipopolysaccharide (LPS) comprising part of the cell wall structure (see Chapter 2), may be released during the normal growth cycles of bacteria or after the destruction of bacteria by host defenses. Endotoxin (or the core of the LPS, lipid A) has been shown to mediate numerous systemic reactions, including a febrile response, and the activation of complement and certain blood-clotting factors. Although gram-positive bacteria do not contain the lipid A endotoxin, many produce exotoxins, and the effects of their presence in the bloodstream may be equally devastating to the patient. Once a vein is selected, the skin site is defatted (fat removal) with 70% isopropyl alcohol and an antiseptic is applied to kill surface and subsurface bacteria. Regardless of the antiseptic used, it is critical to follow the manufacturer’s recommendation for the length of time the antiseptic is allowed to remain on the skin. Available data indicate that iodine tincture (iodine in alcohol) and chlorhexidine are equivalent for skin preparation before drawing blood cultures. The steps necessary for drawing blood for culture are given in Procedure 68-1, which can be found on the Evolve site.
Bloodstream Infections
General Considerations
Etiology
Bacteria
Parasites
Types of Bloodstream Infections
Intravascular Infections
Infective Endocarditis.
Mycotic Aneurysm and Suppurative Thrombophlebitis.
Intravenous Catheter–Associated Bacteremia.
Extravascular Infections
Organism
Extravascular Site of Infection
Anaerobic organisms
Wound, soft tissue
Brucella spp.
Reticuloendothelial system
Candida albicans
Genitourinary tract
Chlamydia pneumoniae
Respiratory
Clostridium spp.
Wound, soft tissue
Coagulase negative staphylococci
Wound, soft tissue
Enterobacteriaceae (E.coli, Klebsiella spp., Enterobacter spp., Proteus spp., Enterococcus spp.)
Genitourinary tract infections, central nervous system
Haemophilus influenzae
Meninges (CNS), epiglotitis, periorbital region, respiratory
Legionella spp.
Respiratory
Listeria monocytogenes
Meninges (CNS)
Neisseria meningitidis
Meninges (CNS)
Pseudomonas aeruginosa
Wound, soft tissue, central nervous system
Salmonella enterica typhi
Small intestine, regional lymph nodes of the intestine, reticuloendothelial system
Streptococcus penumoniae
Meninges (CNS), respiratory
Streptococcus pyogenes
Wound, soft tissue
Staphylococcus aureus
Wound, soft tissue, meninges (CNS)
Clinical Manifestations
Detection of Bacteremia
Specimen Collection
Preparation of the Site
Antisepsis.