Blood
COMPOSITION OF BLOOD
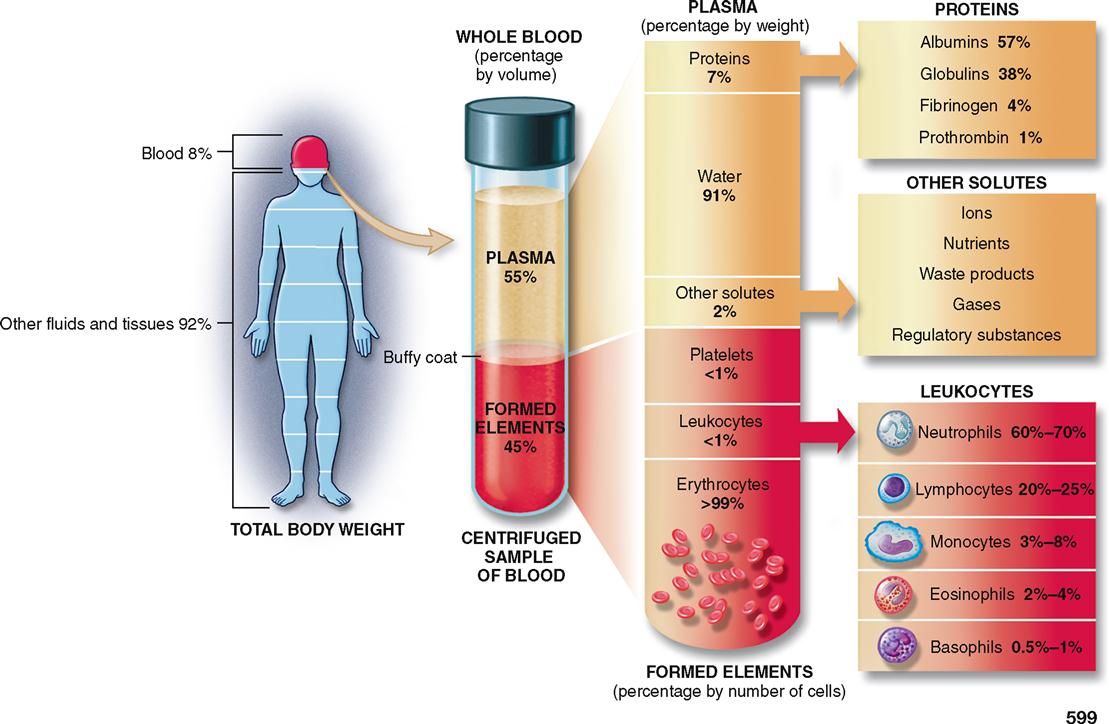
Blood is much more than the simple liquid it seems to be. It consists of not only a fluid but also cells, including thrombocytes (platelets). The fluid portion of blood, that is, the plasma, is one of the three major body fluids (interstitial and intracellular fluids are the other two). As explained below, the quantity of whole blood found in the body (blood volume) is often expressed as a percent of total body weight. However, the measurement of the plasma and formed elements is generally expressed as a percent of the whole blood volume. Using these measurement criteria, whole blood constitutes about 8% of total body weight, plasma accounts for 55%, and the formed elements account for about 45% of the total blood volume (Figure 20-1). The term formed elements is used to designate the various kinds of blood cells that are normally present in blood.
Blood is a complex transport medium that performs vital pickup and delivery services for the body. It picks up food and oxygen from the digestive and respiratory systems and delivers them to cells while also picking up wastes from cells for delivery to excretory organs. Blood also transports hormones, enzymes, buffers, and various other biochemical substances that serve important functions. Blood serves another critical function. It is the keystone of the body’s heat-regulating mechanism. Certain physical properties of blood make it especially effective in this role. Its high specific heat and conductivity enable this unique fluid to absorb large quantities of heat without an appreciable increase in its own temperature and to transfer this absorbed heat from the core of the body to its surface, where the heat can be more readily dissipated (see Figure 7-15, p. 183).
Blood Volume
How much blood does the body contain? The answer is about 8% of total body weight in average-sized adults. In a healthy young female, that amounts to about 4 to 5 liters and in a male about 5 to 6 liters. In addition to gender differences, blood volume varies with age, body composition, and method of measurement. A unit of blood is the amount (0.45 liter or just under 1 pint) that is collected from a blood donor for transfusion purposes. It constitutes about 10% of total blood volume in many adults.
Blood volume can be determined using direct methods or indirect methods of measurement. Direct measurement of total blood volume can be accomplished only by complete removal of all blood from an experimental animal. In humans, indirect methods of measurement that employ “tagging” of red blood cells or plasma components with radioisotopes are used. The principle is simply to introduce a known amount of radioisotope into the circulation, allow the material to be distributed uniformly throughout the blood, and then analyze its concentration in a representative blood sample. Having an accurate measurement is important in replacing blood lost because of hemorrhage or in treating other serious conditions such as shock.
One of the chief variables influencing normal blood volume is the amount of body fat. Blood volume per kilogram of body weight varies inversely with the amount of excess body fat. This means that the less fat there is in your body, the more blood you have per kilogram of your body weight. Because females normally have a higher percent of body fat than males, they have less blood per kilogram of body weight and therefore a lower blood volume.
FORMED ELEMENTS OF BLOOD
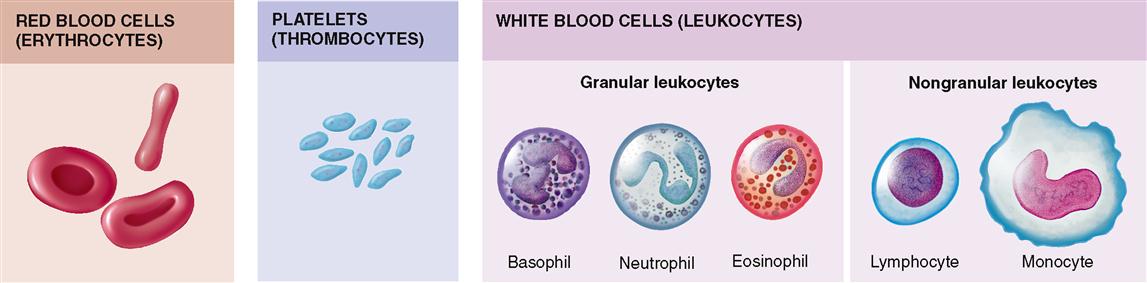
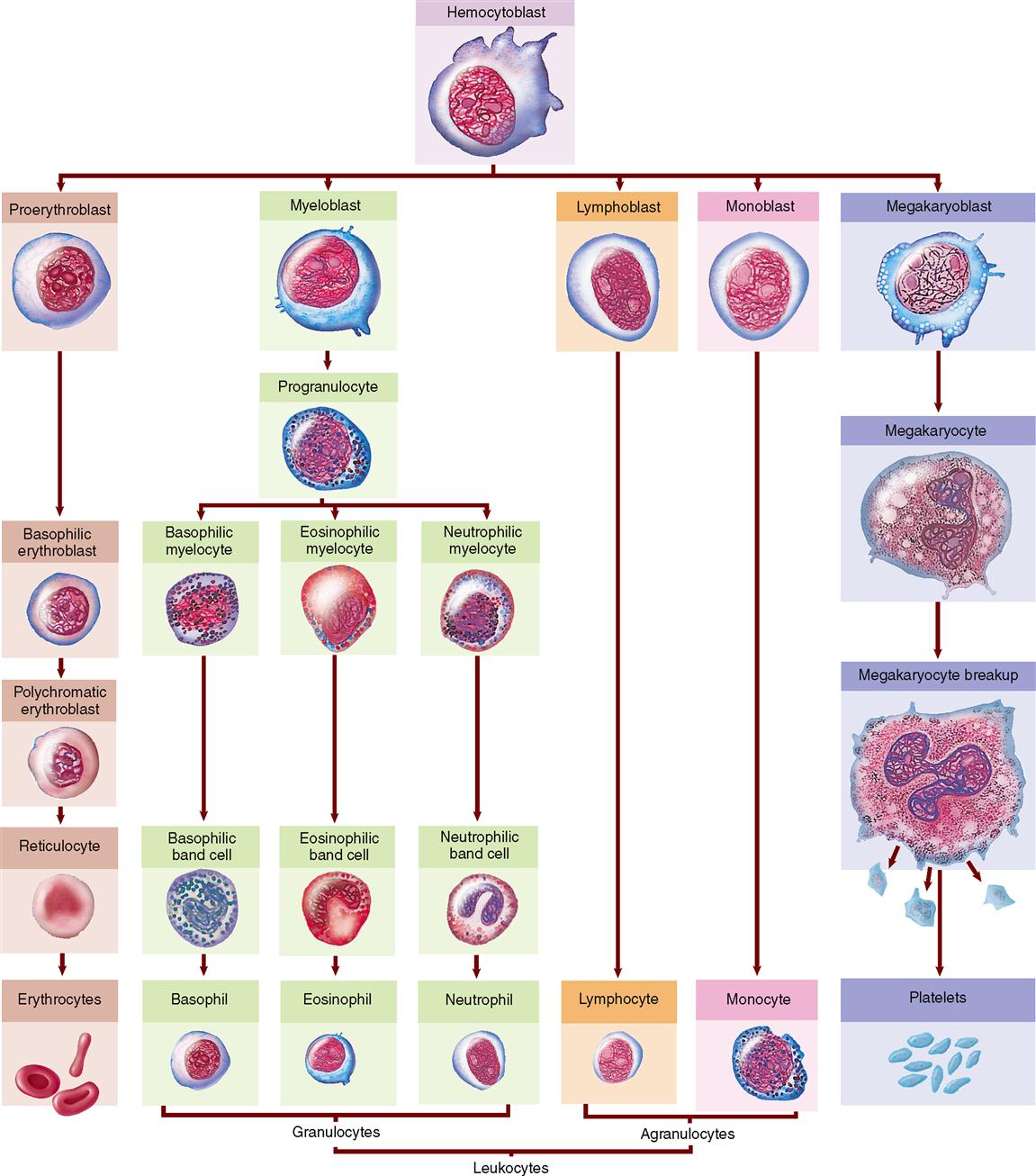
Figure 20-2 illustrates the formed elements of blood. They are as follows:
Plasma, when separated from “whole blood,” is a clear straw-colored fluid that consists of about 90% water and 10% solutes. Blood transfusions most often involve three major blood components—plasma, platelets, and red blood cells. In the United States, red blood cells constitute about 13.5 million transfusions compared with 1.75 million transfusions of platelets and 3.5 million transfusions of plasma each year.
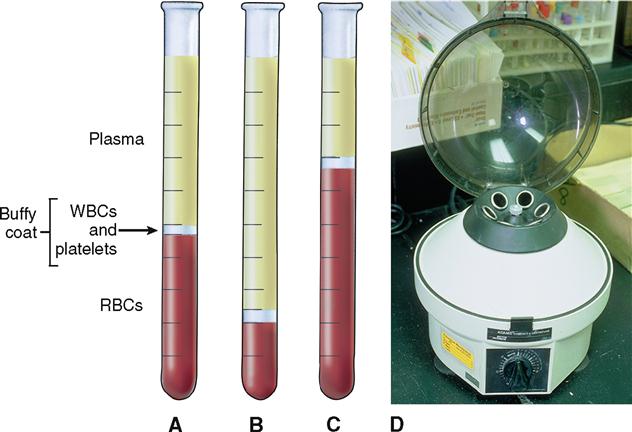
If a tube of whole blood, that is, plasma and formed elements, is allowed to stand or is spun in a centrifuge, separation will occur. The term packed cell volume (PCV), or hematocrit (hee-MAT-oh-krit), is used to describe the volume percent of red blood cells (RBCs) in whole blood. In Figure 20-3, A, a sample of normal whole blood has been separated by spinning in a centrifuge so that the formed elements are forced to the bottom. The percentage of plasma is about 55% of the total sample, whereas the packed cell volume, or hematocrit, is 45%. A hematocrit of 45% means that in every 100 ml of whole blood there are 45 ml of RBCs and 55 ml of fluid plasma.
Normally the average hematocrit for a man is about 45% (40% to 54%, normal range) and for a woman about 42% (38% to 47%, normal range). Conditions that result in decreased RBC numbers (Figure 20-3, B) are called anemias and are characterized by a reduced hematocrit value. Healthy individuals who live and work in high altitudes often have elevated RBC numbers and hematocrit values (Figure 20-3, C). The condition is called physiological polycythemia (from word parts meaning “condition of many blood cells”).
Box 20-1 describes another clinical blood test that involves separating RBCs from plasma.
White blood cells (WBCs), or leukocytes, and platelets make up less than 1% of blood volume. Note in Figure 20-3 that a thin white layer of leukocytes and platelets, called the buffy coat, is present at the interface between the packed red cells and plasma.
Red Blood Cells (Erythrocytes)
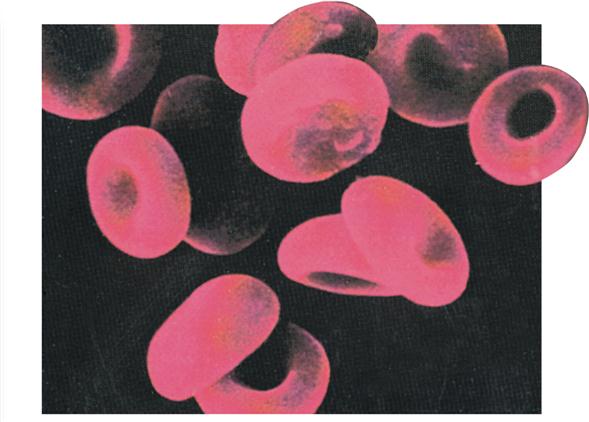
A normal, mature RBC has no nucleus and is only about 7.5 μm in diameter. More than 1500 of them could be placed side by side in a 1 cm space. Before the cell reaches maturity and enters the bloodstream from the bone marrow, the nucleus is extruded. As you can see in Figure 20-4, normal, mature RBCs are shaped like tiny biconcave disks.
The mature erythrocyte is also unique in that it does not contain ribosomes, mitochondria, and other organelles typical of most body cells. Instead, the primary component of each RBC is the red protein pigment, hemoglobin. It accounts for more than one third of the cell volume and is critically important to its primary function.
The depression on each flat surface of the cell results in a thin center and thicker edges. This unique shape of the RBC gives it a very large surface area relative to its volume. The shape of erythrocytes can passively change as they forcibly pass through blood capillaries that often are smaller than the typical 7.5 μm diameter of an erythrocyte.
The flexibility of RBC shape is possible because of the presence of stretchable fibers composed of a unique protein called spectrin. These fibers, which are part of the cytoskeleton, adhere to the inside of the erythrocyte plasma membrane. It is the presence of flexible spectrin fibers that permits the plasma membrane surrounding the RBC to accommodate change from a more typical biconcave shape to a smaller cup-shaped cell size and then back to its normal size and appearance when deforming pressures are no longer being applied to the surface of the plasma membrane. This ability to change shape is necessary for the survival of RBCs, which are under almost constant mechanical shearing and bursting strains as they pass through the capillary system. In addition, the degree of cell deformity that is possible influences the speed of blood flow in the microcirculation.
RBCs are the most numerous of the formed elements in the blood. In men, RBC counts average about 5,500,000 per cubic millimeter (mm3) of blood; in women, 4,800,000/mm3. Gender differences in RBC numbers may be influenced by the stimulating effect of the male sex hormone testosterone on RBC production. RBC numbers in females are normally lower than those in males.
FUNCTION OF RED BLOOD CELLS
RBCs play a critical role in the transport of oxygen and carbon dioxide in the body. Chapter 27 includes a detailed discussion of how oxygen is transported from air in the lungs to the body cells and how carbon dioxide moves from body cells to the lungs for removal. Both of these functions depend on hemoglobin. In addition to hemoglobin, the presence of an enzyme, carbonic anhydrase (CA), in RBCs catalyzes a reaction that joins carbon dioxide and water to form carbonic acid. Dissociation of the acid then generates bicarbonate ions (HCO3−) and hydrogen ions (H+), which diffuse out of the RBCs. Because carbon dioxide (CO2) is chemically incorporated into the newly formed bicarbonate ions, it can be transported in this new form in the blood plasma until it is excreted from the body. Bicarbonate ions also have an important role in maintaining normal blood pH levels (see Chapter 33).
Considered together, the total surface area of all the RBCs in an adult is enormous. It provides an area larger than a football field for the exchange of respiratory gases between hemoglobin found in circulating erythrocytes and interstitial fluid that bathes the body cells. This is an excellent example of the relationship between form and function.
HEMOGLOBIN
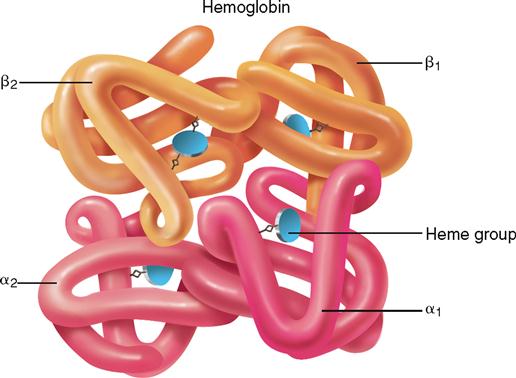
Packed within each RBC are an estimated 200 to 300 million molecules of hemoglobin, which make up about 95% of the dry weight of each cell. Each hemoglobin molecule is composed of four protein chains. Each chain, called a globin, is bound to a red pigment, identified in Figure 20-5, as a heme group. Each heme group contains one iron atom. Therefore one hemoglobin molecule contains four iron atoms. This structural fact enables one hemoglobin molecule to unite with four oxygen molecules to form oxyhemoglobin (a reversible reaction). Hemoglobin can also combine with carbon dioxide to form carbaminohemoglobin (also reversible). But in this reaction the structure of the globin part of the hemoglobin molecule, rather than its heme part, makes the combining possible.
A man’s blood usually contains more hemoglobin than a woman’s. In most normal men, 100 ml of blood contains 14 to 16 grams of hemoglobin. The normal hemoglobin content of a woman’s blood is a little less—specifically, in the range of 12 to 14 grams/100 ml. Recall that testosterone in the male tends to stimulate erythrocyte production and cause an increase in RBC numbers. Increased levels of hemoglobin in males are directly related to increased erythrocyte numbers. An adult who has a hemoglobin content of less than 10 grams/100 ml of blood is diagnosed as having anemia. If you dissect the word parts, you see that anemia literally means “lack of blood.” The term anemia also may be used to describe a reduction in the number or volume of functional RBCs in a given unit of whole blood. Anemias are classified according to the size and hemoglobin content of RBCs. Box 20-2 describes a type of anemia that results from production of an abnormal type of hemoglobin.
FORMATION OF RED BLOOD CELLS
The entire process of RBC formation is called erythropoiesis. In the adult, erythrocytes begin their maturation sequence in the red bone marrow from nucleated cells known as hematopoietic stem cells, or adult blood-forming stem cells. Adult stem cells are cells that have the ability to maintain a constant population of newly differentiating cells of a specific type. These adult blood-forming stem cells divide by mitosis; some of the daughter cells remain as undifferentiated adult stem cells, whereas others go through several stages of development to become erythrocytes. Figure 20-6 shows the developmental stages that can be identified as transformation from the immature forms to the mature red blood cell occurs. The entire maturation process requires about 4 days and proceeds from step to step by gradual transition. Just what influences direct embryonic stem cells (found in the embryo and in umbilical cord blood) to develop into a specific type of adult stem cell (e.g., hematopoietic stem cells) and then eventually differentiate into a specific cell type, such as an RBC, is a topic of intense research.
As you can see in Figure 20-6, all blood cells are derived from hematopoietic stem cells. In RBCs, differentiation begins with the appearance of proerythroblasts. Mitotic divisions then produce basophilic erythroblasts. The next maturation division produces polychromatic erythroblasts, which produce hemoglobin. These cells subsequently lose their nuclei and become reticulocytes. Once released into the circulating blood, reticulocytes lose their delicate reticulum and become mature erythrocytes in about 24 to 36 hours. Note in Figure 20-6 that overall cell size decreases as the maturation sequence progresses.
RBCs are formed and destroyed at a breathtaking rate. Every day of our adult lives we produce more than 200 billion RBCs to replace an equal number destroyed during that brief time. Because in health the number of RBCs remains relatively constant, efficient homeostatic mechanisms must operate to balance the number of cells formed against the number destroyed.
The rate of RBC production soon speeds up if blood oxygen levels reaching the tissues decrease. Oxygen deficiency increases RBC numbers by increasing the secretion of a glycoprotein hormone named erythropoietin (eh-RITH-roh-POY-eh-tin), or EPO. An inactive form of this hormone, called an erythropoietinogen, is released into the blood primarily from the liver on an ongoing basis. If oxygen levels decrease, the kidneys release increasing amounts of erythropoietin, which in turn stimulates bone marrow to accelerate its production of red blood cells. With increasing numbers of RBCs, oxygen delivery to tissues increases, and less erythropoietin is produced, and consequently less is available to stimulate RBC production in the red bone marrow. Figure 20-7 shows how erythropoietin production is controlled by a negative feedback loop that is activated by decreasing oxygen concentration in the tissues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree