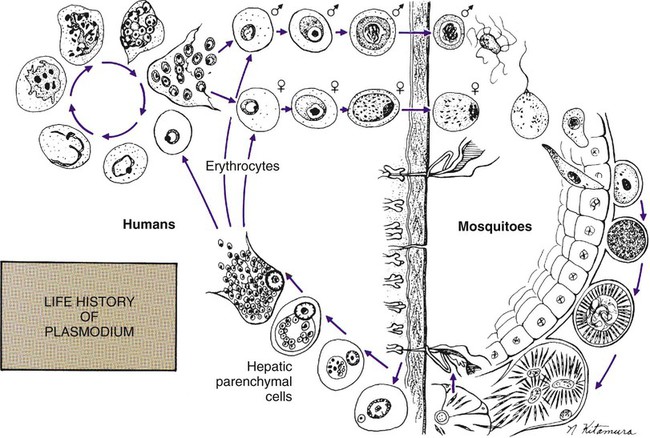
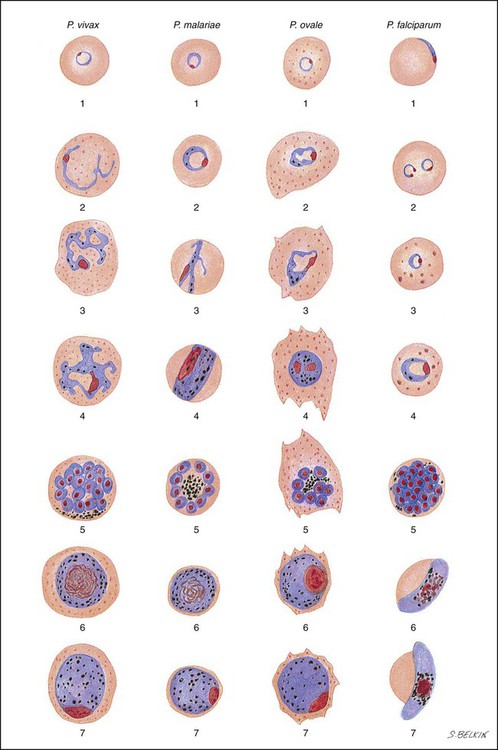
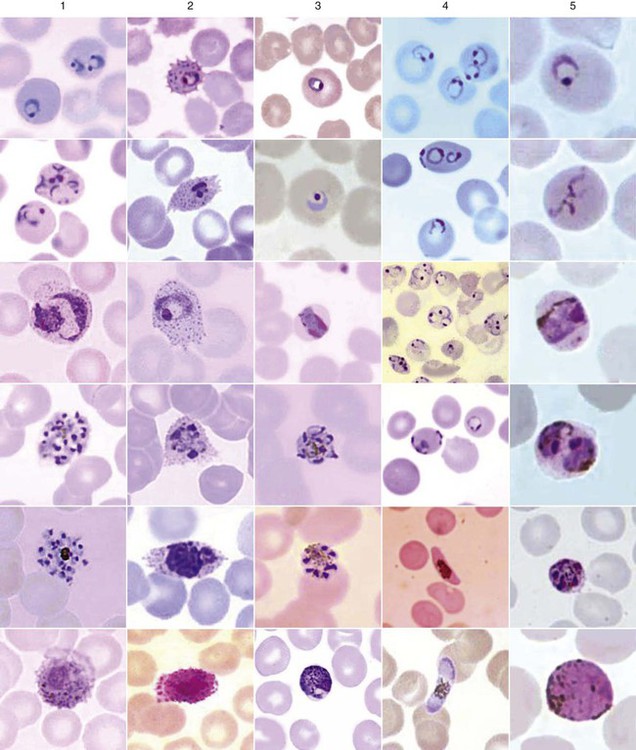
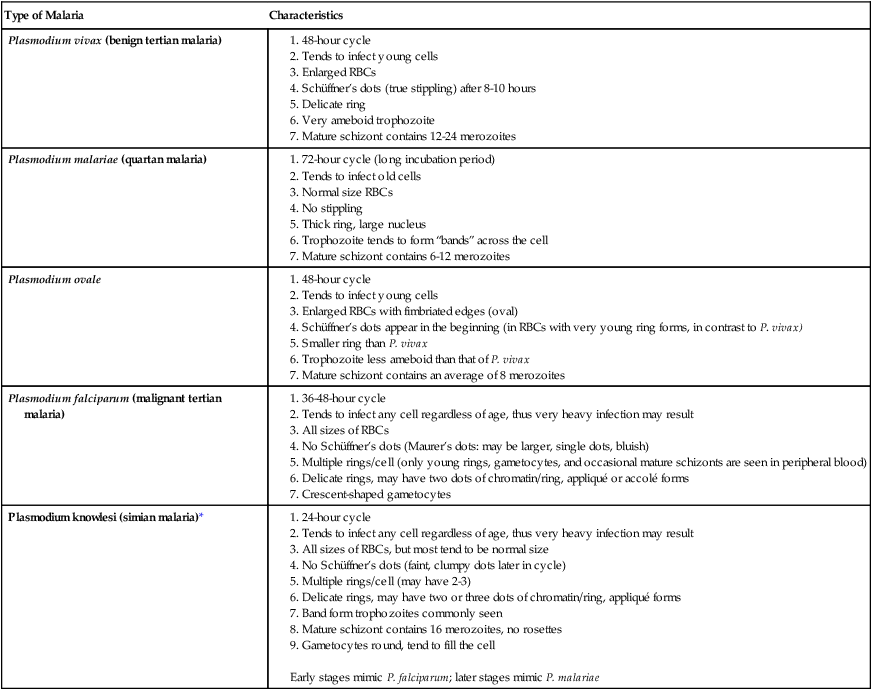
Chapter 49 1. Explain the general life cycle of Plasmodium spp. including both asexual and sexual stages, exoerythrocytic and erythrocytic cycle trophozoites, schizonts, hypnozoites, merozoites, gametocytes, and sporozoites. 2. Describe the distinguishing morphologic characteristics, clinical disease, vectors, stages of infectivity, and laboratory diagnosis for Plasmodium spp., Babesia spp., and Trypanosoma and Leishmania spp. 3. Define paroxysm in malarial periodicity. 4. Compare and contrast recrudescence and relapse including the physiologic basis for each during infection with malaria. 5. Compare and contrast the pathogenesis of infections with P. falciparum, P. malariae, P. ovale, P. vivax, and P. knowlesi including variation in signs and symptoms. 6. Differentiate intracellular forms of Babesia spp. from Plasmodium spp. 7. Define and describe the life cycle stages of Trypanosoma and Leishmania spp. including amastigotes, promastigotes, trypomastigotes, epimastigotes, and metacyclic trypanosome forms when appropriate. The vector for malaria is the female anopheline mosquito. When the vector takes a blood meal, sporozoites contained in the salivary glands of the mosquito are discharged into the puncture wound (Figure 49-1). Within an hour, these infective sporozoites are carried via the blood to the liver, where they penetrate hepatocytes and begin to grow, initiating the preerythrocytic or primary exoerythrocytic cycle. The sporozoites become round or oval and begin dividing repeatedly. Schizogony results in large numbers of exoerythrocytic merozoites. Once these merozoites leave the liver, they invade the red blood cells (RBCs), initiating the erythrocytic cycle. A dormant schizogony may occur in P. vivax and P. ovale organisms, which remain quiescent in the liver. These resting stages have been termed hypnozoites and lead to a true relapse, often within 1 year or up to more than 5 years later. Delayed schizogony does not occur in P. falciparum, P. malariae, or P. knowlesi. P. vivax infects only the reticulocytes; thus, the parasitemia is limited to approximately 2% to 5% of the available RBCs (Tables 49-1 to 49-3, Figures 49-2 and 49-3). Splenomegaly occurs during the first few weeks of infection, and the spleen will progress from being soft and palpable to hard, with continued enlargement during a chronic infection. If the infection is treated during the early phases, the spleen will return to its normal size. A secondary or dormant schizogony occurs in P. vivax and P. ovale, which remain quiescent in the liver. These resting stages have been termed hypnozoites. TABLE 49-1 Plasmodium spp.: Clinical Characteristics of the Five Human Infections TABLE 49-2 Plasmodia in Giemsa-Stained Thin Blood Smears TABLE 49-3 Malaria Characteristics with Fresh Blood or Blood Collected Using EDTA with No Extended Lag Time* *Preparation of thick and thin blood films within <60 min of collection. After a few days of irregular periodicity, a regular 48-hour cycle is established. An untreated primary attack may last from 3 weeks to 2 months or longer. Over time, the paroxysms (symptomatic period) become less severe and more irregular in frequency and then cease altogether. In approximately 50% of patients infected with P. vivax, relapses occur after weeks, months, or even after 5 years or more. The RBCs tend to be enlarged (young RBCs), there may be Schüffner’s dots (exclusively found in P. vivax and P. ovale) after 8 to 10 hours, the developing rings are ameboid, and the mature schizont contains 12 to 24 merozoites (Figure 49-3 [3]). Although P. ovale and P. vivax infections are clinically similar, P. ovale malaria is usually less severe, tends to relapse less frequently, and usually ends with spontaneous recovery, often after no more than 6 to 10 paroxysms (see Tables 49-1 to 49-3, Figures 49-2 and 49-3). Like P. vivax, P. ovale infects only the reticulocytes, so that the parasitemia is limited to approximately 2% to 5% of the available RBCs. For many years the literature has stated that as with P. vivax, a secondary or dormant schizogony occurs in P. ovale, which remain quiescent in the liver. However, newer findings indicate that hypnozoites have never been demonstrated by biologic experiments.
Blood and Tissue Protozoa
Plasmodium spp.
Plasmodium Vivax (Benign Tertian Malaria)
General Characteristics
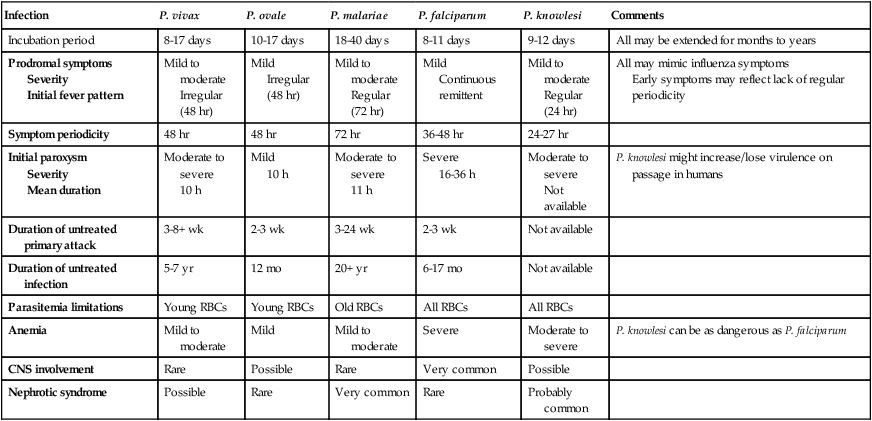
Infection
P. vivax
P. ovale
P. malariae
P. falciparum
P. knowlesi
Comments
Incubation period
8-17 days
10-17 days
18-40 days
8-11 days
9-12 days
All may be extended for months to years
Prodromal symptoms
Severity
Initial fever pattern
Mild to moderate
Irregular (48 hr)
Mild
Irregular (48 hr)
Mild to moderate
Regular (72 hr)
Mild
Continuous remittent
Mild to moderate
Regular (24 hr)
All may mimic influenza symptoms
Early symptoms may reflect lack of regular periodicity
Symptom periodicity
48 hr
48 hr
72 hr
36-48 hr
24-27 hr
Initial paroxysm
Severity
Mean duration
Moderate to severe
10 h
Mild
10 h
Moderate to severe
11 h
Severe
16-36 h
Moderate to severe
Not available
P. knowlesi might increase/lose virulence on passage in humans
Duration of untreated primary attack
3-8+ wk
2-3 wk
3-24 wk
2-3 wk
Not available
Duration of untreated infection
5-7 yr
12 mo
20+ yr
6-17 mo
Not available
Parasitemia limitations
Young RBCs
Young RBCs
Old RBCs
All RBCs
All RBCs
Anemia
Mild to moderate
Mild
Mild to moderate
Severe
Moderate to severe
P. knowlesi can be as dangerous as P. falciparum
CNS involvement
Rare
Possible
Rare
Very common
Possible
Nephrotic syndrome
Possible
Rare
Very common
Rare
Probably common
Plasmodium vivax
Plasmodium malariae
Plasmodium falciparum
Plasmodium ovale
Plasmodium knowlesi
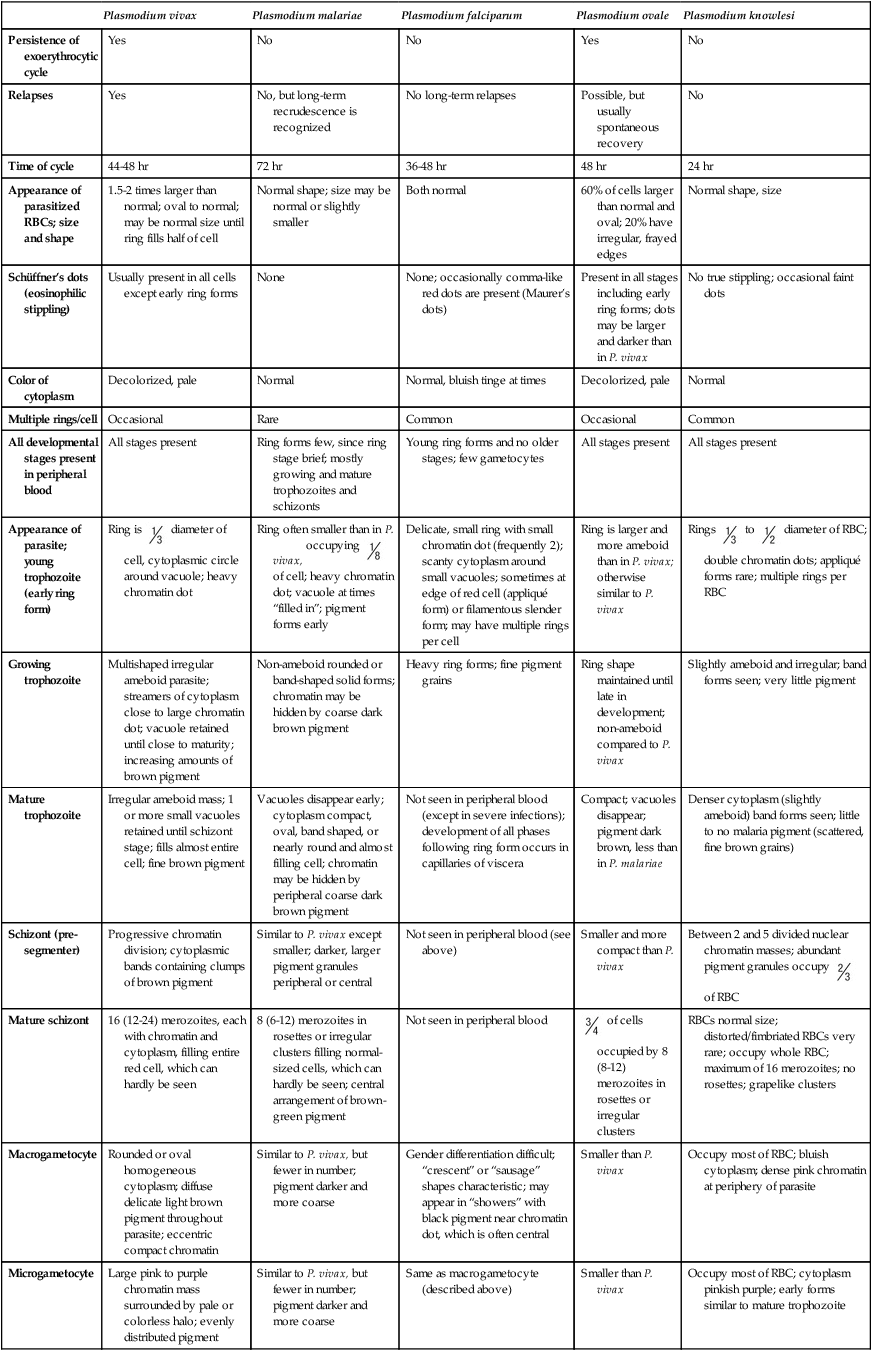
Persistence of exoerythrocytic cycle
Yes
No
No
Yes
No
Relapses
Yes
No, but long-term recrudescence is recognized
No long-term relapses
Possible, but usually spontaneous recovery
No
Time of cycle
44-48 hr
72 hr
36-48 hr
48 hr
24 hr
Appearance of parasitized RBCs; size and shape
1.5-2 times larger than normal; oval to normal; may be normal size until ring fills half of cell
Normal shape; size may be normal or slightly smaller
Both normal
60% of cells larger than normal and oval; 20% have irregular, frayed edges
Normal shape, size
Schüffner’s dots (eosinophilic stippling)
Usually present in all cells except early ring forms
None
None; occasionally comma-like red dots are present (Maurer’s dots)
Present in all stages including early ring forms; dots may be larger and darker than in P. vivax
No true stippling; occasional faint dots
Color of cytoplasm
Decolorized, pale
Normal
Normal, bluish tinge at times
Decolorized, pale
Normal
Multiple rings/cell
Occasional
Rare
Common
Occasional
Common
All developmental stages present in peripheral blood
All stages present
Ring forms few, since ring stage brief; mostly growing and mature trophozoites and schizonts
Young ring forms and no older stages; few gametocytes
All stages present
All stages present
Appearance of parasite; young trophozoite (early ring form)
Ring is 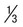 diameter of cell, cytoplasmic circle around vacuole; heavy chromatin dot
diameter of cell, cytoplasmic circle around vacuole; heavy chromatin dot
Ring often smaller than in P. vivax, occupying 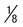 of cell; heavy chromatin dot; vacuole at times “filled in”; pigment forms early
of cell; heavy chromatin dot; vacuole at times “filled in”; pigment forms early
Delicate, small ring with small chromatin dot (frequently 2); scanty cytoplasm around small vacuoles; sometimes at edge of red cell (appliqué form) or filamentous slender form; may have multiple rings per cell
Ring is larger and more ameboid than in P. vivax; otherwise similar to P. vivax
Rings 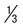 to
to 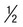 diameter of RBC; double chromatin dots; appliqué forms rare; multiple rings per RBC
diameter of RBC; double chromatin dots; appliqué forms rare; multiple rings per RBC
Growing trophozoite
Multishaped irregular ameboid parasite; streamers of cytoplasm close to large chromatin dot; vacuole retained until close to maturity; increasing amounts of brown pigment
Non-ameboid rounded or band-shaped solid forms; chromatin may be hidden by coarse dark brown pigment
Heavy ring forms; fine pigment grains
Ring shape maintained until late in development; non-ameboid compared to P. vivax
Slightly ameboid and irregular; band forms seen; very little pigment
Mature trophozoite
Irregular ameboid mass; 1 or more small vacuoles retained until schizont stage; fills almost entire cell; fine brown pigment
Vacuoles disappear early; cytoplasm compact, oval, band shaped, or nearly round and almost filling cell; chromatin may be hidden by peripheral coarse dark brown pigment
Not seen in peripheral blood (except in severe infections); development of all phases following ring form occurs in capillaries of viscera
Compact; vacuoles disappear; pigment dark brown, less than in P. malariae
Denser cytoplasm (slightly ameboid) band forms seen; little to no malaria pigment (scattered, fine brown grains)
Schizont (pre-segmenter)
Progressive chromatin division; cytoplasmic bands containing clumps of brown pigment
Similar to P. vivax except smaller; darker, larger pigment granules peripheral or central
Not seen in peripheral blood (see above)
Smaller and more compact than P. vivax
Between 2 and 5 divided nuclear chromatin masses; abundant pigment granules occupy 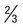 of RBC
of RBC
Mature schizont
16 (12-24) merozoites, each with chromatin and cytoplasm, filling entire red cell, which can hardly be seen
8 (6-12) merozoites in rosettes or irregular clusters filling normal-sized cells, which can hardly be seen; central arrangement of brown-green pigment
Not seen in peripheral blood
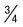 of cells occupied by 8 (8-12) merozoites in rosettes or irregular clusters
of cells occupied by 8 (8-12) merozoites in rosettes or irregular clusters
RBCs normal size; distorted/fimbriated RBCs very rare; occupy whole RBC; maximum of 16 merozoites; no rosettes; grapelike clusters
Macrogametocyte
Rounded or oval homogeneous cytoplasm; diffuse delicate light brown pigment throughout parasite; eccentric compact chromatin
Similar to P. vivax, but fewer in number; pigment darker and more coarse
Gender differentiation difficult; “crescent” or “sausage” shapes characteristic; may appear in “showers” with black pigment near chromatin dot, which is often central
Smaller than P. vivax
Occupy most of RBC; bluish cytoplasm; dense pink chromatin at periphery of parasite
Microgametocyte
Large pink to purple chromatin mass surrounded by pale or colorless halo; evenly distributed pigment
Similar to P. vivax, but fewer in number; pigment darker and more coarse
Same as macrogametocyte (described above)
Smaller than P. vivax
Occupy most of RBC; cytoplasm pinkish purple; early forms similar to mature trophozoite
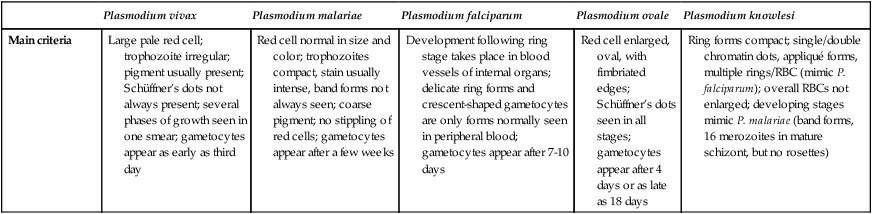
Main criteria
Large pale red cell; trophozoite irregular; pigment usually present; Schüffner’s dots not always present; several phases of growth seen in one smear; gametocytes appear as early as third day
Red cell normal in size and color; trophozoites compact, stain usually intense, band forms not always seen; coarse pigment; no stippling of red cells; gametocytes appear after a few weeks
Development following ring stage takes place in blood vessels of internal organs; delicate ring forms and crescent-shaped gametocytes are only forms normally seen in peripheral blood; gametocytes appear after 7-10 days
Red cell enlarged, oval, with fimbriated edges; Schüffner’s dots seen in all stages; gametocytes appear after 4 days or as late as 18 days
Ring forms compact; single/double chromatin dots, appliqué forms, multiple rings/RBC (mimic P. falciparum); overall RBCs not enlarged; developing stages mimic P. malariae (band forms, 16 merozoites in mature schizont, but no rosettes)
Plasmodium Ovale
General Characteristics
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Blood and Tissue Protozoa