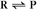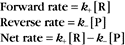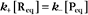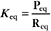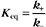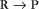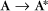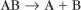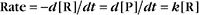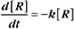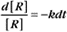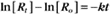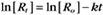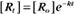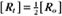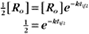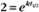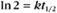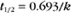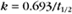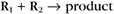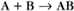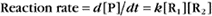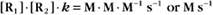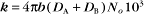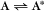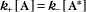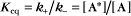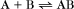CHAPTER 4 Biophysical Principles*
The concepts in this chapter form the basis for understanding all the molecular interactions in chemistry and biology. To illustrate some of these concepts with a practical example, the chapter concludes with a section on an exceptionally important family of enzymes that bind and hydrolyze the nucleotide GTP. This example provides the background knowledge to understand how GTPases participate in numerous processes covered in later chapters.
The extent of chemical reactions is characterized by the equilibrium constant; the rates of these reactions are described by rate constants. This chapter reviews the physical basis for rate constants and how they are related to the thermodynamic parameter, the equilibrium constant. These simple but powerful principles permit a deeper appreciation of molecular interactions in cells. On the basis of many examples presented in this book, it will become clear to the reader that rate constants are at least as important as equilibrium constants, since the rates of reactions govern the dynamics of the cell. The chapter includes discussion of the chemical bonds important in biochemistry. Box 4-1 lists key terms used in this chapter.
BOX 4-1 Key Biophysical Terms
Reaction rates are expressed as follows:
At equilibrium, the forward rate equals the reverse rate:
and concentrations of reactants Req and products Peq do not change with time.
First-Order Reactions
First-order reactions have one reactant (R) and produce a product (P). The general case is simply
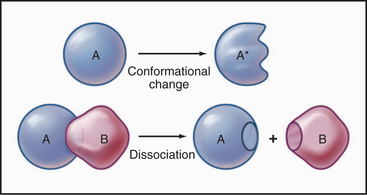
Some common examples of first-order reactions (Fig. 4-1) include conformational changes, such as a change in shape of protein A to shape A*:
and the dissociation of complexes, such as
A first-order rate constant can be viewed as a probability per unit of time. For a conformational change, it is the probability that any A will change to * in a unit of time. For dissociation of complex AB, the first-order rate constant is determined by the strength of the bonds holding the complex together. This “dissociation rate constant” can be viewed as the probability that the complex will fall apart in a unit of time. The probability of the conformational change of any particular A to * or of the dissociation of any particular AB is independent of its concentration. The concentra-tions of A and AB are important only in determining the rate of the reaction observed in a bulk sample (Box 4-2).
BOX 4-2 Relationship of the Half-Time to a First-Order Rate Constant
where Ro is the initial concentration and Rt is the concentration at time t. Rearranging, we have
When the initial concentration Ro is reduced by half,
To review, the rate of a first-order reaction is simply the product of a constant that is characteristic of the reaction and the concentration of the single reactant. The constant can be calculated from the half-time of a reaction (Box 4-2).
Second-Order Reactions
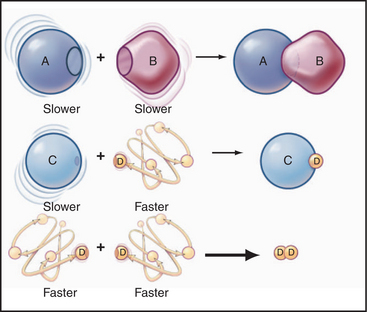
Second-order reactions have two reactants (Fig. 4-2). The general case is
A common example in biology is a bimolecular association reaction, such as
the same as a first-order reaction.
The value of a second-order “association” rate constant, k+, is determined mainly by the rate at which the molecules collide. This collision rate depends on the rate of diffusion of the molecules (Fig. 4-2), which is determined by the size and shape of the molecule, the viscosity of the medium, and the temperature. These factors are summarized in a parameter called the diffusion coefficient, D, with units of m2 s−1. D is a measure of how fast a molecule moves in a given medium. The rate constant for collisions is described by the Debye-Smoluchowski equation, a relationship that depends only on the diffusion coefficients and the area of interaction between the molecules: