Objectives
- List approximate percentages of sodium reabsorbed in major tubular segments.
- List approximate percentages of water reabsorbed in major tubular segments.
- Describe proximal tubule sodium reabsorption, including the functions of the apical membrane sodium entry mechanisms and the basolateral Na-K-ATPase.
- Explain why chloride reabsorption is coupled with sodium reabsorption, and list the major pathways of proximal tubule chloride reabsorption.
- State the maximum and minimum values of urine osmolality.
- Define osmotic diuresis and water diuresis.
- Explain why there is always an obligatory water loss.
- Describe the handling of sodium by the descending and ascending limbs, distal tubule, and collecting-duct system.
- Describe the role of sodium-potassium-2 chloride symporters in the thick ascending limb.
- Describe the handling of water by descending and ascending limbs, distal tubule, and collecting-duct system.
- Describe the process of “separating salt from water” and why this is required to excrete either concentrated or dilute urine.
- Describe how antidiuretic hormone affects water and urea reabsorption.
- Describe the characteristics of the medullary osmotic gradient.
- Explain the role of the thick ascending limb, urea recycling, and medullary blood flow in generating the medullary osmotic gradient.
- State why the medullary osmotic gradient is partially “washed out” during a water diuresis.
Overview
This and Chapter 7 are devoted entirely to the renal handling of sodium, chloride, and water. Sodium and chloride are crucial substances because they account for most of the osmotic content of the extracellular fluid while water constitutes the major part of the body volume. And water is the solvent for all dissolved solutes. As described in Chapter 7, these substances play a huge role in the function of the cardiovascular system and are subject to complex regulation.
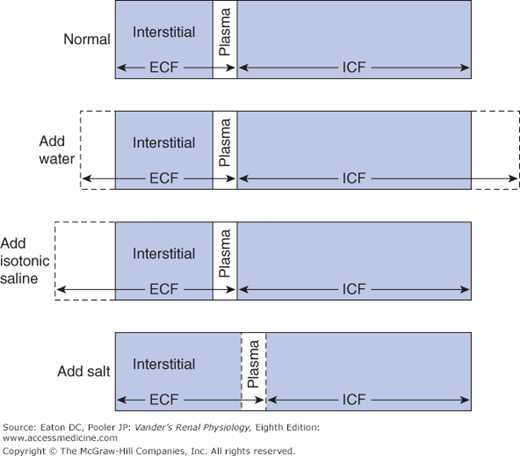
![]() Approximately 60% of the body weight is made up of water, which is distributed into various aqueous spaces in proportion to their osmotic content. The collective volume of all the cells in the body is called the intracellular fluid (ICF). It contains roughly two thirds of the body osmotic content, and therefore two thirds of the water. The remaining one third of the osmotic content and water is called the extracellular fluid (ECF). It is mostly interstitial fluid and blood plasma. Because of the ease with which water crosses most cell membranes (see Chapter 4) the ECF and ICF are in osmotic equilibrium. The total of the 2 volumes varies with gain and loss of water, whereas the relative proportion in each compartment is influenced by gain and loss of sodium. Additions or losses of sodium from the body are mostly to or from the ECF because the actions of cellular Na-K-ATPases prevent major changes in intracellular sodium concentration.1 If the addition or loss of fluid is isotonic sodium, only the volume of the ECF is affected, but if the fluid is either hyper- or hypo-osmotic, both compartments change volume. These events are depicted in Figure 6–1. The addition of water alone expands both the ICF and ECF (indicated by the dashed lines). Adding sodium chloride without water does not change the total volume, but causes a shift of water from the ICF to the ECF in order to restore equality of osmolality between the 2 compartments.
Approximately 60% of the body weight is made up of water, which is distributed into various aqueous spaces in proportion to their osmotic content. The collective volume of all the cells in the body is called the intracellular fluid (ICF). It contains roughly two thirds of the body osmotic content, and therefore two thirds of the water. The remaining one third of the osmotic content and water is called the extracellular fluid (ECF). It is mostly interstitial fluid and blood plasma. Because of the ease with which water crosses most cell membranes (see Chapter 4) the ECF and ICF are in osmotic equilibrium. The total of the 2 volumes varies with gain and loss of water, whereas the relative proportion in each compartment is influenced by gain and loss of sodium. Additions or losses of sodium from the body are mostly to or from the ECF because the actions of cellular Na-K-ATPases prevent major changes in intracellular sodium concentration.1 If the addition or loss of fluid is isotonic sodium, only the volume of the ECF is affected, but if the fluid is either hyper- or hypo-osmotic, both compartments change volume. These events are depicted in Figure 6–1. The addition of water alone expands both the ICF and ECF (indicated by the dashed lines). Adding sodium chloride without water does not change the total volume, but causes a shift of water from the ICF to the ECF in order to restore equality of osmolality between the 2 compartments.
![]() Sodium, chloride, and water are all freely filterable at the renal corpuscle. They all undergo considerable tubular reabsorption, (usually more than 99%!), but normally no tubular secretion. Most of the renal ATP energy expended every day is used to accomplish this enormous reabsorptive task. In terms of transport mechanisms, the transport of water is the simplest. As we pointed out in Chapter 4, “water follows the osmoles.” Thus, much of the description for water transport really amounts to describing solute transport, taking into account the fact that in some regions of the kidney the epithelium has low water permeability, leaving water confined to the tubular lumen even though solute is removed. Transport of chloride involves more steps, but is often passive and, because of the constraints of electroneutrality, tied to the transport of sodium. Sodium transport is the most complicated. First, its transport is linked to the transport of many other substances, and second, its rates of transport at various places are subject to regulation by multiple controls. However, if we keep in mind the generalized model of epithelial transport developed in Chapter 4 (Figure 4–4) it is not difficult to grasp the key features of sodium transport.
Sodium, chloride, and water are all freely filterable at the renal corpuscle. They all undergo considerable tubular reabsorption, (usually more than 99%!), but normally no tubular secretion. Most of the renal ATP energy expended every day is used to accomplish this enormous reabsorptive task. In terms of transport mechanisms, the transport of water is the simplest. As we pointed out in Chapter 4, “water follows the osmoles.” Thus, much of the description for water transport really amounts to describing solute transport, taking into account the fact that in some regions of the kidney the epithelium has low water permeability, leaving water confined to the tubular lumen even though solute is removed. Transport of chloride involves more steps, but is often passive and, because of the constraints of electroneutrality, tied to the transport of sodium. Sodium transport is the most complicated. First, its transport is linked to the transport of many other substances, and second, its rates of transport at various places are subject to regulation by multiple controls. However, if we keep in mind the generalized model of epithelial transport developed in Chapter 4 (Figure 4–4) it is not difficult to grasp the key features of sodium transport.
The inputs, and therefore excretory rates, of sodium, chloride, and water vary over an extremely wide range. For example, some persons may ingest 20 to 25 g of sodium chloride per day, whereas a person on a low-salt diet may ingest only 0.05 g. The normal kidney can readily alter its excretion of salt over this range. Similarly, urinary water excretion can be varied from approximately 0.4 to 25 L/day, depending on whether one is lost in the desert or drinking excessive water for nonphysiological reasons.
Roughly 2/3 of the filtered sodium, chloride, and water are reabsorbed in the proximal tubule in all conditions |
Table 6–1 is a balance sheet for sodium chloride. Clearly the major route of salt excretion from the body under normal circumstances is via the kidneys. The large amount excreted should not obscure the fact that nearly all the filtered sodium is reabsorbed. Table 6–2 summarizes the approximate quantitative contribution of each tubular segment to sodium reabsorption. In an individual with an average salt intake, the proximal tubule reabsorbs about 65% of the filtered sodium, the thin and thick ascending limbs of Henle’s loop about 25%, and the distal convoluted tubule and collecting-duct system most of the remaining 10%, so that the final urine contains less than 1% of the total filtered sodium. As discussed in Chapter 7, reabsorption at several of these tubular sites is under physiological control by multiple signals, so that the exact amount of sodium excreted is homeostatically regulated. Because so much sodium is filtered, even a small percentage change in reabsorption results in a relatively large change in excretion.
| Percent of filtered load reabsorbed (%) | ||
|---|---|---|
| Tubular segment | Sodium | Water |
| Proximal tubule | 65 | 65 |
| Descending thin limb of Henle’s loop | — | 10 |
| Ascending thin limb and thick ascending limb of Henle’s loop | 25 | — |
| Distal convoluted tubule | 5 | — |
| Collecting-duct system | 4–5 | 5 (during water-loading) >24 (during dehydration) |
![]() In all nephron segments, the essential event for active transcellular sodium reabsorption is the primary active transport of sodium from cell to interstitial fluid by the Na-K-ATPase pumps in the basolateral membrane. These pumps keep the intracellular sodium concentration lower than in the surrounding media. In addition, the inside of the cell is negatively charged with respect to the lumen, and luminal sodium ions enter the cell passively, down both their concentration and electrical gradients.
In all nephron segments, the essential event for active transcellular sodium reabsorption is the primary active transport of sodium from cell to interstitial fluid by the Na-K-ATPase pumps in the basolateral membrane. These pumps keep the intracellular sodium concentration lower than in the surrounding media. In addition, the inside of the cell is negatively charged with respect to the lumen, and luminal sodium ions enter the cell passively, down both their concentration and electrical gradients.
The tubular locations that reabsorb chloride and the percentages of filtered chloride reabsorbed by these segments are similar to those for sodium because of the constraints of electroneutrality (see Table 6–1). Any finite volume of fluid must contain equal amounts of anion and cation equivalents. One liter of normal filtrate contains 140 mEq of sodium and approximately 140 mEq of anions, mainly chloride (110 mEq) and bicarbonate (24 mEq). (We say “approximately” because there are other cations [eg, potassium and calcium] and anions [eg, sulfate and phosphate], but their contributions are much smaller than sodium, chloride, and bicarbonate.) If 65% of the sodium in 1 liter of filtrate is reabsorbed in the proximal tubule (0.65 × 140 = 91 mEq), then electroneutrality requires that 91 mEq of some combination of chloride and bicarbonate must also be reabsorbed in the proximal tubule to accompany this sodium. As described in Chapter 9, approximately 90% of the filtered bicarbonate is reabsorbed in the proximal tubule (0.9 × 24 ≈ 22). This leaves 91 – 22 = 69 mEq of chloride that must be reabsorbed in the proximal tubule. This is more than 60% of the filtered chloride and very similar to the fractional reabsorption of sodium. Later segments reabsorb almost all of the remaining 40%.
There is both passive paracellular chloride reabsorption as well as active transcellular reabsorption. In active transcellular chloride reabsorption, the critical transport step for chloride is usually from lumen to cell. The chloride transport process in the luminal membrane must go against the negative membrane potential that repels anions, and it must achieve a high enough intracellular chloride concentration to drive downhill chloride movement out of the cell across the basolateral membrane. Thus, luminal membrane chloride transporters serve essentially the same function for chloride that the basolateral membrane Na-K-ATPase pumps do for sodium: they use energy to move chloride uphill from lumen to cell against its electrochemical gradient.
A balance sheet for total body water is given in Table 6–3. These are average values, which are subject to considerable variation. The 2 sources of body water are metabolically produced water, resulting largely from the oxidation of carbohydrates, and ingested water obtained from liquids and so-called solid food (eg, a rare steak is approximately 70% water). There are several sites from which water is always lost to the external environment: skin, lungs, gastrointestinal tract, and kidneys. Menstrual flow and, in lactating women, breast milk constitute 2 other potential sources of water loss in women.
The loss of water by evaporation from the cells of the skin and the lining of respiratory passageways is a continuous process, often referred to as insensible loss because people are unaware of its occurrence. Additional water evaporates from the skin during production of sweat. Fecal water loss is normally quite small but can be severe in diarrhea. Gastrointestinal loss can also be large during severe bouts of vomiting. Under conditions of normal hydration, the kidneys are, of course, the main route of water loss.
![]() With a large water load, the renal response is to produce a large volume of very dilute urine (osmolality much lower than in blood plasma). In contrast, during a state of dehydration, the urine volume is low and very concentrated (ie, the urine osmolality is much greater than in blood plasma). That the urine osmolality is so variable brings us to a crucial aspect of renal function. Terrestrial animals must be able to independently control excretion of salt and water, because their ingestion and loss is not always linked (see Tables 6–1 and 6–3). To excrete water in excess of salt and vice versa (ie, produce a range of urine osmolalities), the kidneys must be able to separate the reabsorption of solute from the reabsorption of water, that is, to “separate salt from water,” a process described later in this chapter. Water reabsorption always occurs in the proximal tubule (65% of the filtered water), descending thin limb of Henle’s loop (10%), and collecting-duct system (where the fractional reabsorption is highly variable). A comparison of water and sodium reabsorption (see Table 6–2) reveals several important points. First, sodium and water reabsorption occur in the proximal tubule to the same extent. Second, both are also reabsorbed in Henle’s loop, but not in equal proportions. The part of the loop involved in water reabsorption is different than for sodium reabsorption, and the fraction of sodium reabsorbed by the loop as a whole is always greater than that of water (ie, the loop overall is a site where salt is reabsorbed and excess water is left in the lumen of the nephron: “separating salt from water”). By the time tubular fluid leaves the loop of Henle and enters the distal tubule, the loss of solute has typically decreased the osmolality to only one third of the plasma value. Third, sodium reabsorption, but not water reabsorption, occurs in the distal convoluted tubule. Fourth, both occur in the collecting-duct system, but the percentages of sodium and water reabsorbed in the collecting-duct system vary enormously depending on body conditions.
With a large water load, the renal response is to produce a large volume of very dilute urine (osmolality much lower than in blood plasma). In contrast, during a state of dehydration, the urine volume is low and very concentrated (ie, the urine osmolality is much greater than in blood plasma). That the urine osmolality is so variable brings us to a crucial aspect of renal function. Terrestrial animals must be able to independently control excretion of salt and water, because their ingestion and loss is not always linked (see Tables 6–1 and 6–3). To excrete water in excess of salt and vice versa (ie, produce a range of urine osmolalities), the kidneys must be able to separate the reabsorption of solute from the reabsorption of water, that is, to “separate salt from water,” a process described later in this chapter. Water reabsorption always occurs in the proximal tubule (65% of the filtered water), descending thin limb of Henle’s loop (10%), and collecting-duct system (where the fractional reabsorption is highly variable). A comparison of water and sodium reabsorption (see Table 6–2) reveals several important points. First, sodium and water reabsorption occur in the proximal tubule to the same extent. Second, both are also reabsorbed in Henle’s loop, but not in equal proportions. The part of the loop involved in water reabsorption is different than for sodium reabsorption, and the fraction of sodium reabsorbed by the loop as a whole is always greater than that of water (ie, the loop overall is a site where salt is reabsorbed and excess water is left in the lumen of the nephron: “separating salt from water”). By the time tubular fluid leaves the loop of Henle and enters the distal tubule, the loss of solute has typically decreased the osmolality to only one third of the plasma value. Third, sodium reabsorption, but not water reabsorption, occurs in the distal convoluted tubule. Fourth, both occur in the collecting-duct system, but the percentages of sodium and water reabsorbed in the collecting-duct system vary enormously depending on body conditions.
Water can cross the tubular epithelium by several routes. Small amounts can move by simple diffusion through the lipid bilayer, but not enough to be significant. The majority moves through aquaporins in plasma membranes of the tubular cells and through the tight junctions between the cells. The amount of water that moves for a given osmotic gradient and its route depends on the water permeability of the different cellular components. The basolateral membranes of all renal cells are quite permeable to water due to the presence of aquaporins. As a result, the cytosolic osmolality is always close to that of the surrounding interstitium. It is the luminal membrane and tight junctions where most of the variability lies. The segments of the renal tubule fall into several general categories with regard to water permeability: (1) Only in the proximal tubule are the tight junctions significantly permeable to water. The luminal membranes of the proximal tubule cells are also highly permeable to water. (2) The luminal membranes of the early parts of the descending thin limb of Henle’s loop also have a very high water permeability. (3) The luminal membranes of the ascending limbs of Henle’s loop (both thin and thick; recall from Chapter 1 that only long loops have ascending thin limbs) and the luminal membranes of the distal convoluted tubule are always relatively water impermeable, as are the tight junctions. (4) The water permeability of the luminal membranes of the collecting-duct system is intrinsically low but can be regulated so that it increases substantially. These differences in water permeability account for the sites of water reabsorption as well as the large range of water reabsorption given for the collecting-duct system in Table 6–2.
The ability of the kidneys to produce low-volume hyperosmotic urine is a major determinant of one’s ability to survive without water, which for most people is several days, and even longer under optimal conditions. The human kidney can produce a maximal urinary concentration of 1400 mOsm/kg in extreme dehydration. This is almost 5 times the osmolality of plasma. The sum of the urea, sulfate, phosphate, other waste products, and a small number of nonwaste ions excreted each day normally averages approximately 600 mOsm/day. Therefore, the minimum volume of water in which this mass of solute can be dissolved is roughly 600 mmol/1400 mOsm/L = 0.43 L/day.
This volume of urine is known as the obligatory water loss. It is not a strictly fixed value but changes with different physiological states. For example, increased tissue catabolism, as during fasting or trauma, releases excess solute and so increases obligatory water loss.
The obligatory water loss contributes to dehydration when a person is deprived of water and limits survival time. For example, if we could produce urine with an osmolarity of 6000 mOsm/L, the obligatory water loss would only be 100 mL of water, and survival time would be greatly increased. A desert rodent, the kangaroo rat, does just that. This animal does not need to drink water because the water content of its food and the water produced by metabolism of the foods is sufficient to meet its needs.2
1Besides the sodium dissolved in the body fluids there is a considerable amount of sodium in the mineral component of bone that is not osmotically active. In addition, the polysaccharides of connective tissue loosely bind sodium in nonosmotic form.
2The obligatory solute excretion explains why a thirsty sailor cannot drink seawater, even if the urine osmolality is slightly greater than that of the seawater. To excrete all the salt in 1 L of seawater (to prevent a net gain of salt) plus the obligatory organic solutes produced by the body, the volume of urine would have to be much greater than 1 L.
Individual Tubular Segments
The important principles to be understood regarding individual tubular segments are (1) the mechanisms for reabsorption of sodium, chloride, and water, (2) how they relate to one another, and (3) how the amount of reabsorption quantitatively varies from one segment to another.
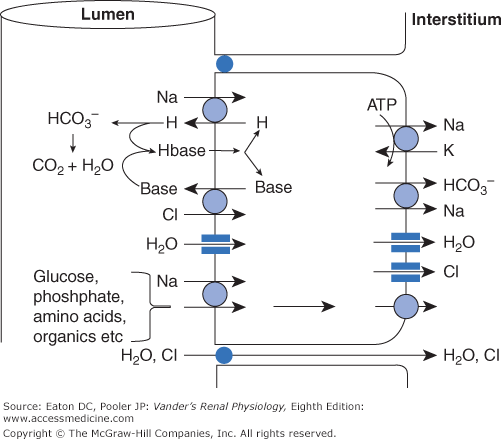
As shown in Figure 6–2, sodium enters proximal tubule cells by several luminal entry steps. In the early portion, a large fraction of the tubular sodium enters the cells across the luminal membrane via antiport with protons from within the cells. Given the large amount of sodium reabsorbed compared with the vanishingly low levels of cellular protons, how can there be enough protons to supply the transporter? Furthermore, what happens to all those protons once in the lumen? These issues will be described thoroughly in Chapter 9, but for now we note that protons are generated continuously by combining carbon dioxide with water, a process that produces protons and bicarbonate. The protons exit the cell across the luminal membrane in exchange for sodium entry, while bicarbonate exits the cell across the basolateral membrane in symport with sodium. Many of the secreted protons combine with filtered bicarbonate to form carbon dioxide and water once again. Therefore, in the early proximal tubule, bicarbonate is a major anion reabsorbed with sodium, and the luminal bicarbonate concentration decreases markedly (Figure 6–3). The other secreted protons combine with other secreted bases as described below. Organic nutrients such as glucose are also absorbed with sodium, and their luminal concentrations decrease rapidly.
Figure 6–2
Major pathways for reabsorption of sodium, chloride, and water in the proximal tubule. The entire proximal tubule is the major site for reabsorption of salt and water. Sodium entry is coupled to the secretion or uptake of a variety of substances, the major one being secreted hydrogen ions via the NHE-3 antiporter. These hydrogen ions combine with filtered bicarbonate and secreted organic base (see text and Chapter 9 for further explanation). Additional sodium enters in symport with glucose, amino acids, and phosphate. Sodium is transported to the interstitium mostly via the basolateral Na-K-ATPase, but also in symport with bicarbonate. (The coupling between sodium and bicarbonate is described fully in Chapter 9.) Chloride that enters in antiport with organic base leaves mostly via channels. In addition, a substantial amount of chloride is reabsorbed paracellularly. Water moves both paracellularly and intracellularly via aquaporins. ATP, adenosine triphosphate.