Chapter One. Basic biochemistry
Energy
The production, storage and release of energy are essential to living cells which need a constant supply of energy to function and reproduce. This energy is acquired from breakdown of food molecules, in particular sugars. There are two main types of energy: kinetic and potential. Kinetic energy is the energy of movement and includes thermal (heat) energy. Potential or stored energy is more relevant to biological systems. Glucose stores potential energy and is broken down continuously to perform work (Guyton & Hall 2006). Adenosine triphosphate (ATP) is important in energy release (Ch. 23).
Catabolic reactions (breakdown of cell products) release large quantities of energy, whereas anabolic reactions (such as the manufacture of proteins) are energy-requiring. Cells must have a balance between energy-producing and energy-demanding processes (Rose 1999). All forms of energy are interchangeable and can be expressed in the same unit of measurement. The SI unit (International System of Units) for measuring energy is the joule (J) or kilojoule (kJ): 1 kJ = 4.2 calories.
The chemistry of living organisms
Atoms
Living organisms are made up of chemical elements. Over 100 elements are known and each has its own symbol. These elements form a periodic table depending on the atomic mass of each element (see below). Elements consist of particles called atoms, which are the smallest indivisible part of an element that still retain its chemical and physical properties. Atoms are constructed from three subatomic particles: neutrons, protons and electrons (Sackheim 2008). The central nucleus of the atom is made up of neutrons and protons of similar mass, and the very small electrons are arranged in orbital shells surrounding the nucleus (Fig. 1.1).
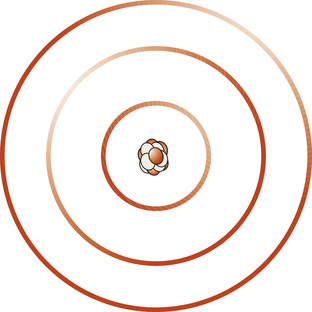 |
| Figure 1.1 Diagrammatic representation of the structure of an atom showing the nucleus surrounded by electron orbital shells. (From Montague S E, Watson R, Herbert R A 2005, with kind permission of Elsevier.) |
The formation of particles within the atom is maintained by minute electrical charges. The neutrons of the nucleus carry no charge, protons carry a positive charge and electrons carry a negative charge. The number of protons is equal to the number of electrons so that most atoms are uncharged. Each element has a different number of electrons and protons which give it its atomic number. Neutrons are heavy and contribute to the mass of the element. The number of neutrons and protons together give the element its mass number. This determines the atomic mass ( atomic weight) of an element. Table 1.1 gives values for the six most common elements which make up 99% of living matter.
| Element | Atomic number | Number of protons | Number of neutrons | Mass number | Atomic mass |
|---|---|---|---|---|---|
| Hydrogen | 1 | 1 | 0 | 1 | 1 |
| Carbon | 6 | 6 | 6 | 12 | 12 |
| Nitrogen | 7 | 7 | 7 | 14 | 14 |
| Oxygen | 8 | 8 | 8 | 16 | 16 |
| Phosphorus | 15 | 15 | 16 | 31 | 31 |
| Calcium | 20 | 20 | 20 | 40 | 40 |
Radioactive atoms
Variation in the number of neutrons in an atom leads to different forms of the element called isotopes, with different mass numbers. In some isotopes the presence of extra neutrons causes them to be unstable. They will break down into a more stable configuration ( decay) during which they radiate energy and atomic particles. This is radioactivity and the isotopes are radioactive. Radioactive stable isotopes have been used successfully in medical diagnosis and treatment (Cooper 2006).
Molecules
Atoms are formed into molecules by chemical bonds of which there are two types: the strong, stable covalent bond which is hard to disrupt, and the weaker, less-stable non-covalent bond. The making and breaking of these bonds is associated with energy changes; the more stable the bond, the greater the thermal energy needed to disrupt it. These bonds are formed by electrons that can be donated, received or shared by atoms. One bond is formed by one electron, but some atoms have more than one electron that is free to form bonds. The number of available electrons is called the valency of the atom. For example, hydrogen has a valency of 1 and carbon a valency of 4.
Covalent bonds
When atoms are joined together by sharing electrons a molecule is formed by covalent bonds. The atoms are held closely together because electrons in their outermost shells move in orbitals that are shared by both atoms. Some atoms require more than one electron to form a bond with another atom. Bonds may be single, such as in a molecule of hydrogen gas, or double, as in a molecule of oxygen gas. Complex molecules are formed by linkage of different atoms depending on their valencies. Molecules can be represented as a molecular formula or structure (Table 1.2).
| Atomic element | Valency | Compound | Molecular formula | Molecular structure | Bond type |
|---|---|---|---|---|---|
| H | 1 | Hydrogen gas | H 2 |  | Single |
| O | 2 | Oxygen gas | O 2 | 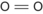 | Double |
| O | 2 | Water | H 2O | 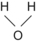 | Single |
| N | 3 | Nitrogen gas | N 2 | 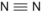 | Triple |
| N | 3 | Ammonia | NH 3 | 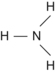 | Single |
| C | 4 | Carbon dioxide | CO 2 | 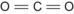 | Double |
| C | 4 | Methane | CH 4 | 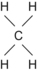 | Single |
| P | 5 |



