OBJECTIVES
After studying this chapter, you should be able to:
Describe the location of the cell bodies and axonal trajectories of preganglionic and postganglionic sympathetic and parasympathetic neurons.
Name the neurotransmitters that are released by preganglionic autonomic neurons, postganglionic sympathetic neurons, postganglionic parasympathetic neurons, and adrenal medullary cells.
Name the types of receptors on autonomic ganglia and on various target organs and list the ways that drugs can act to alter the function of the processes involved in transmission within the autonomic nervous system.
Describe functions of the sympathetic and parasympathetic nervous systems.
Describe the location of some forebrain and brainstem neurons that are components of central autonomic pathways.
Describe the composition and functions of the enteric nervous system.
INTRODUCTION
The autonomic nervous system (ANS) is the part of the nervous system that is responsible for homeostasis. Except for skeletal muscle, which gets its innervation from the somatomotor nervous system, innervation to all other organs is supplied by the ANS. Nerve terminals are located in smooth muscle (eg, blood vessels, the wall of the gastrointestinal tract, and urinary bladder), cardiac muscle, and glands (eg, sweat glands and salivary glands). Although survival is possible without an ANS, the ability to adapt to environmental stressors and other challenges is severely compromised. The importance of understanding the functions of the ANS is underscored by the fact that so many drugs used to treat a vast array of diseases exert their actions on elements of the ANS. Also, many neurologic diseases or disorders result directly from a loss of preganglionic sympathetic neurons (eg, multiple system atrophy and Shy–Drager syndrome) and other common diseases (eg, Parkinson disease and diabetes) are associated with autonomic dysfunction (Clinical Box 13–1).
The ANS has two major and anatomically distinct divisions: the sympathetic and parasympathetic nervous systems. As will be described, some target organs are innervated by both divisions and others are controlled by only one. In addition, the ANS includes the enteric nervous system within the gastrointestinal tract. The classic definition of the ANS is the preganglionic and postganglionic neurons within the sympathetic and parasympathetic divisions. This would be equivalent to defining the somatomotor nervous system as the cranial and spinal motor neurons. A modern definition of the ANS takes into account the descending pathways from several forebrain and brainstem regions as well as visceral afferent pathways that set the level of activity in sympathetic and parasympathetic nerves. This is analogous to including the many descending and ascending pathways that influence the activity of somatic motor neurons as elements of the somatomotor nervous system.
CLINICAL BOX 13–1 Multiple System Atrophy & Shy–Drager Syndrome
Multiple system atrophy (MSA) is a neurodegenerative disorder associated with autonomic failure due to loss of preganglionic autonomic neurons in the spinal cord and brainstem. In the absence of an autonomic nervous system, it is difficult to regulate body temperature, fluid and electrolyte balance, and blood pressure. In addition to these autonomic abnormalities, MSA presents with cerebellar, basal ganglia, locus coeruleus, inferior olivary nucleus, and pyramidal tract deficits. MSA is defined as “a sporadic, progressive, adult-onset disorder characterized by autonomic dysfunction, parkinsonism, and cerebellar ataxia in any combination.” Shy–Drager syndrome is a subtype of MSA in which autonomic failure dominates. The pathologic hallmark of MSA is cytoplasmic and nuclear inclusions in oligodendrocytes and neurons in central motor and autonomic areas. There is also depletion of monoaminergic, cholinergic, and peptidergic markers in several brain regions and in the cerebrospinal fluid. The cause of MSA remains elusive, but there is some evidence that a neuroinflammatory mechanism causing activation of microglia and production of toxic cytokines may occur in brains of MSA patients. Basal levels of sympathetic activity and plasma norepinephrine levels are normal in MSA patients, but they fail to increase in response to standing or other stimuli and leads to severe orthostatic hypotension. In addition to the fall in blood pressure, orthostatic hypotension leads to dizziness, dimness of vision, and even fainting. MSA is also accompanied by parasympathetic dysfunction, including urinary and sexual dysfunction. MSA is most often diagnosed in individuals between 50 and 70 years of age; it affects more men than women. Erectile dysfunction is often the first symptom of the disease. There are also abnormalities in baroreceptor reflex and respiratory control mechanisms. Although autonomic abnormalities are often the first symptoms, 75% of patients with MSA also experience motor disturbances.
THERAPEUTIC HIGHLIGHTSThere is no cure for MSA but various therapies are used to treat specific signs and symptoms of the disease. Corticosteroids are often prescribed to retain salt and water to increase blood pressure. In some individuals, parkinsonian-like signs can be alleviated by administration of levodopa and carbidopa (Sinemet). Various clinical trials are underway to test the effectiveness of using intravenous immunoglobulins to counteract the neuroinflammatory process that occurs in MSA; fluoxetine (a serotonin uptake inhibitor) to prevent orthostatic hypotension, improve mood, and alleviate sleep, pain, and fatigue in MSA patients; and rasagiline (a monoamine oxidase inhibitor) in MSA patients with parkinsonism.
ANATOMIC ORGANIZATION OF AUTONOMIC OUTFLOW
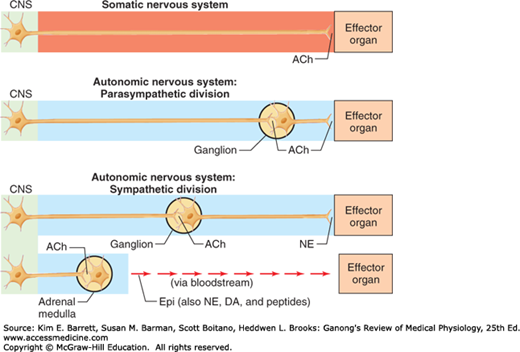
Figure 13–1 compares some fundamental characteristics of the innervation to skeletal muscles with innervation to smooth muscle, cardiac muscle, and glands. As discussed in earlier chapters, the final common pathway linking the central nervous system (CNS) to skeletal muscles is the α-motor neuron. Similarly, sympathetic and parasympathetic neurons serve as the final common pathway from the CNS to visceral targets. However, in marked contrast to the somatomotor nervous system, the peripheral motor portions of the ANS are made up of two neurons: preganglionic and postganglionic neurons. The cell bodies of the preganglionic neurons are located in the intermediolateral (IML) column of the spinal cord and in motor nuclei of some cranial nerves. In contrast to the large diameter and rapidly conducting α-motor neurons, preganglionic axons are small-diameter, myelinated, relatively slowly conducting B fibers. A preganglionic axon diverges to an average of eight or nine postganglionic neurons. In this way, autonomic output is diffuse. The axons of the postganglionic neurons are mostly unmyelinated C fibers and terminate on the visceral effectors.
FIGURE 13–1
Comparison of peripheral organization and transmitters released by somatomotor and autonomic nervous systems. In the case of the somatomotor nervous system, the neuron that leaves the spinal cord projects directly to the effector organ. In the case of the autonomic nervous system, there is a synapse between the neuron that leaves the spinal cord and the effector organ (except for neurons that innervate the adrenal medulla). Note that all neurons that leave the central nervous system release acetylcholine (ACh). DA, dopamine; Epi, epinephrine; NE, norepinephrine. (Used with permission from Widmaier EP, Raff H, Strang KT: Vander’s Human Physiology. New York, NY: McGraw-Hill; 2008.)
One similar feature of autonomic preganglionic neurons and α-motor neurons is that acetylcholine is released at their nerve terminals (Figure 13–1). This is the neurotransmitter released by all neurons whose axons exit the CNS, including cranial motor neurons, α-motor neurons, γ-motor neurons, preganglionic sympathetic neurons, and preganglionic parasympathetic neurons. Postganglionic parasympathetic neurons also release acetylcholine, whereas postganglionic sympathetic neurons release either norepinephrine or acetylcholine.
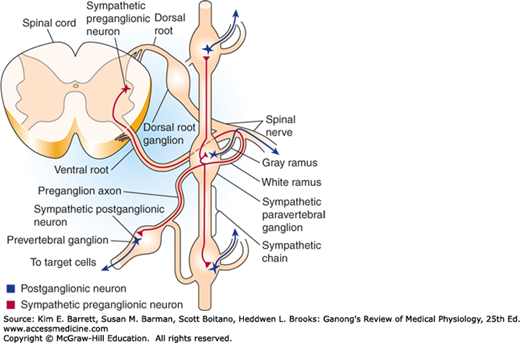
In contrast to α-motor neurons, which are located at all spinal segments, sympathetic preganglionic neurons are located in the IML of only the first thoracic to the third or fourth lumbar segments. This is why the sympathetic nervous system is sometimes called the thoracolumbar division of the ANS. The axons of the sympathetic preganglionic neurons leave the spinal cord at the level at which their cell bodies are located and exit via the ventral root along with axons of α- and γ-motor neurons (Figure 13–2). They then separate from the ventral root via the white rami communicans and project to the adjacent sympathetic paravertebral ganglion, where some of them end on the cell bodies of the postganglionic neurons. Paravertebral ganglia are located adjacent to each thoracic and upper lumbar spinal segment; in addition, there are a few ganglia adjacent to the cervical and sacral spinal segments. The ganglia are connected to each other via the axons of preganglionic neurons that travel rostrally or caudally to terminate on postganglionic neurons located at some distance. Together these ganglia and axons form the sympathetic chain bilaterally. This arrangement is seen in Figure 13–2 and Figure 13–3.
Some preganglionic neurons pass through the paravertebral ganglion chain and end on postganglionic neurons located in prevertebral (or collateral) ganglia close to the viscera, including the celiac, superior mesenteric, and inferior mesenteric ganglia (Figure 13–3). There are also preganglionic neurons whose axons terminate directly on the effector organ, the adrenal gland.
The axons of some of the postganglionic neurons leave the chain ganglia and reenter the spinal nerves via the gray rami communicans and are distributed to autonomic effectors in the areas supplied by these spinal nerves (Figure 13–2). These postganglionic sympathetic nerves terminate mainly on smooth muscle (eg, blood vessels and hair follicles) and on sweat glands in the limbs. Other postganglionic fibers leave the chain ganglia to enter the thoracic cavity to terminate in visceral organs. Postganglionic fibers from prevertebral ganglia also terminate in visceral targets.
The parasympathetic nervous system is sometimes called the craniosacral division of the ANS because of the location of its preganglionic neurons; preganglionic neurons are located in several cranial nerve nuclei (III, VII, IX, and X) and in the IML of the sacral spinal cord (Figure 13–3). The cell bodies in the Edinger–Westphal nucleus of the oculomotor nerve project to the ciliary ganglia to innervate the sphincter (constrictor) muscle of the iris and the ciliary muscle. Neurons in the superior salivatory nucleus of the facial nerve project to the sphenopalatine ganglia to innervate the lacrimal glands and nasal and palatine mucous membranes and to the submandibular ganglia to innervate the submandibular (also called submaxillary) and sublingual glands. The cell bodies in the inferior salivatory nucleus of the glossopharyngeal nerve project to the otic ganglion to innervate the parotid salivary gland. Vagal preganglionic fibers synapse on ganglia cells clustered within the walls of visceral organs; thus these parasympathetic postganglionic fibers are very short. Neurons in the nucleus ambiguus innervate the sinoatrial (SA) and atrioventricular (AV) nodes in the heart; and neurons in the dorsal motor vagal nucleus innervate the esophagus, trachea, lungs, and gastrointestinal tract. The parasympathetic sacral outflow (pelvic nerve) supplies the pelvic viscera via branches of the second to fourth sacral spinal nerves.
CHEMICAL TRANSMISSION AT AUTONOMIC JUNCTIONS
The first evidence for chemical neurotransmission was provided by a simple yet dramatic study by Otto Loewi in 1920 in which he showed that the slowing of the heart rate produced by stimulation of the vagal parasympathetic nerves was due to the release of acetylcholine (see Chapter 7). Transmission at the synaptic junctions between preganglionic and postganglionic neurons and between the postganglionic neurons and the autonomic effectors are chemically mediated. The principal transmitter agents involved are acetylcholine and norepinephrine. The autonomic neurons that are cholinergic (ie, release acetylcholine) are (1) all preganglionic neurons, (2) all parasympathetic postganglionic neurons, (3) sympathetic postganglionic neurons that innervate sweat glands, and (4) sympathetic postganglionic neurons that end on blood vessels in some skeletal muscles and produce vasodilation when stimulated (sympathetic vasodilator nerves). The remaining sympathetic postganglionic neurons are noradrenergic (ie, release norepinephrine). The adrenal medulla is essentially a sympathetic ganglion in which the postganglionic cells have lost their axons and secrete both norepinephrine and epinephrine directly into the bloodstream.
Table 13–1 shows the types of cholinergic and adrenergic receptors at various junctions within the ANS. The junctions in the peripheral autonomic motor pathways are logical sites for pharmacologic manipulation of visceral function. The transmitter agents are synthesized, stored in the nerve endings, and released near the neurons, muscle cells, or gland cells where they bind to various ion channels or G-protein-coupled receptors (GPCR) to initiate their characteristic actions. The neurotransmitters are then removed from the area by reuptake or metabolism. Each of these steps can be stimulated or inhibited, with predictable consequences. Table 13–2 lists how various drugs can affect neurotransmission in autonomic neurons and effector sites.
| Sympathetic Nervous System | |||
|---|---|---|---|
| Effector Organs | Parasympathetic Nervous System | Receptor Type | Response |
| Eyes | |||
Radial muscle of iris Sphincter muscle of iris Ciliary muscle | — Contraction (miosis) Contraction for near vision | α1 | Contraction (mydriasis) — — |
| Heart | |||
SA node Atria and ventricle AV node and Purkinje fibers | Decreased heart rate Decreased atrial contractility Decreased conduction velocity | β1 β1, β2 β1 | Increased heart rate Increased contractility Increased conduction velocity |
| Arterioles | |||
Skin, splanchnic vessels Skeletal muscle | — — |
||