Atypical Small Acinar Proliferations
Gladell P. Paner, MD
Rafael E. Jimenez, MD
Jesse K. McKenney, MD
Key Facts
Terminology
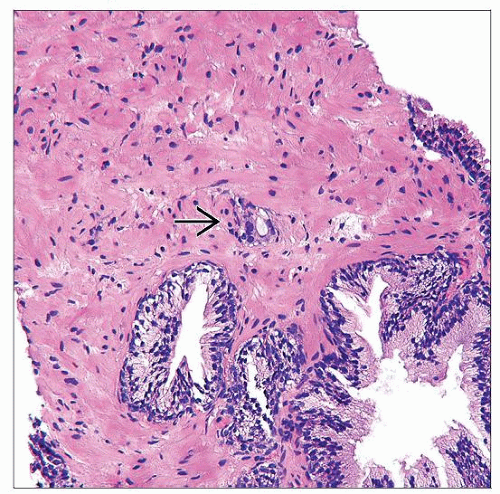
Focus of atypical glands in needle biopsy
Quantitatively &/or qualitatively insufficient for definitive diagnosis or exclusion of prostatic carcinoma
Not a diagnostic entity
Includes undersampled cancer and various benign mimics
Represents descriptive diagnosis to aid in subsequent clinical management
Clinical Issues
Reported in 5% of prostate needle biopsy (0.7-9%)
ASAP diagnosis should prompt rebiopsy
Particularly in area with previous suspicious focus
Carries higher risk of finding PCa in rebiopsy
Mean: 40%; range: 17-70%
Microscopic Pathology
Atypical small glands that either are too few or do not show minimum morphologic features for definite diagnosis as PCa
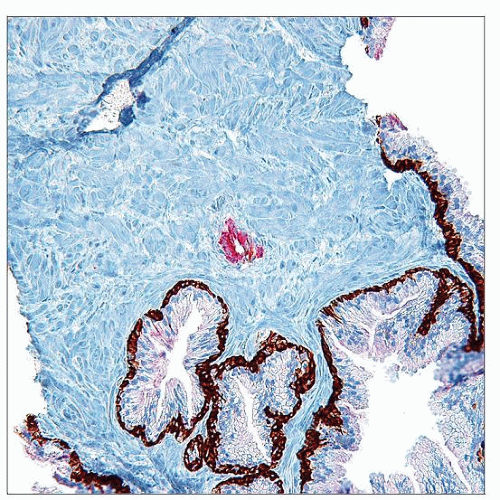
IHC with AMACR and basal cell markers may be helpful
Should be aware of overlap with benign lesions in small foci
Top Differential Diagnoses
Benign
Partial atrophy
Atypical adenomatous hyperplasia (adenosis)
Crowded benign glands
Malignant
Prostatic acinar adenocarcinoma
TERMINOLOGY
Abbreviations
Atypical small acinar proliferation (ASAP)
Synonyms
Suspicious for cancer
Focal glandular atypia
Atypical glands
Definitions
Focus of atypical glands in needle biopsy quantitatively &/or qualitatively insufficient for definitive diagnosis or exclusion of prostate carcinoma (PCa)
ASAP is not an entity
Represents descriptive diagnosis to guide subsequent clinical management
Includes undersampled PCa and various benign mimics of PCa
ASAP introduced in 1993 and is well-accepted descriptive diagnosis in contemporary practice
Term ASAP not entirely accurate as some atypical foci may not be “acinar,” “small,” or “proliferative”
Some atypical glandular lesions suspicious for carcinoma are comprised of large caliber glands (e.g., pseudohyperplastic carcinoma)
CLINICAL ISSUES
Epidemiology
Incidence
Reported in 5% of prostate needle biopsies (individual series vary from 0.7-9%)
Threshold for establishing PCa diagnosis has interobserver variability
Variation, even amongst experts, dependent on experience and training
Presentation
Biopsy performed for known indications
Elevated serum PSA level
Abnormal digital rectal examination
Abnormal transrectal ultrasound
Laboratory Tests
Serum PSA level may be elevated
No significant difference in serum PSA level between patients with PCa on initial biopsy and with ASAP diagnosis preceding subsequent PCa on rebiopsy
Treatment
Patient with ASAP diagnosis should be rebiopsied with extended sampling of prostate gland, including anatomic region where suspicious focus was found
Prognosis
Carries higher risk of finding PCa in rebiopsy (40-50%; individual series vary between 17-70%)
Subsequent PCa may also be identified in contralateral side (up to 27%)
When adjacent to high-grade prostatic intraepithelial neoplasia (HGPIN) (HGPIN and ASAP), higher risk of carcinoma than HGPIN alone
Most PCa detected on subsequent rebiopsy are Gleason score 6 (up to 80%)
May be ≥ Gleason score 8 in some cases (up to 10%)
MICROSCOPIC PATHOLOGY
Histologic Features
Typically group of small crowded glands that do not meet threshold for definitive carcinoma diagnosis
May have too few glands or insufficient qualitative features
Quantitative factors associated with ASAP diagnosis
Inadequate number of glands
No absolute cut-off in number of glands is a formal criteria for diagnosis of PCa
In absence of pathognomonic features, minimum requirement for PCa varies between expert genitourinary pathologists
Most authors do not recommend diagnosis of PCa on single atypical gland; others favor presence of at least 3 glands
Threshold largely dependent on extent of other associated qualitative features: Infiltrative growth, cytologic atypia, intraluminal mucin, crystalloids, nucleoli
Small size of atypical glandular focus
Linear extent usually < 0.8 mm
Qualitative factors associated with ASAP diagnosis
Features may suggest PCa but are insufficient to reach diagnostic threshold for PCa
Architecture may not show definite infiltration
May lack significant nuclear atypia (i.e., absence of nucleomegaly &/or nucleolomegaly)
Glandular lumina may have smooth, sharp contour (no luminal irregularity or infolding) or basal palisading of nuclei without other features
Diagnostic threshold for PCa may be more stringent when certain histologic findings are present: Atrophic features, pseudohyperplastic appearance, or foamy cytoplasm
ASAP frequently does not have any pathognomonic features of PCa
Glomerulations
Collagenous micronodules (mucinous fibroplasia)
Circumferential perineural or intraneural invasion
Invasion of adipose tissue or seminal vesicle
Confounding features
Presence at edge of biopsy
Poor cytologic detail, such as crush artifact
Obscuring inflammation
Loss of atypical focus on subsequent (and intervening) levels precluding adjunctive immunohistochemistry (IHC)
When adjacent to HGPIN, makes distinction from noninvasive outpouchings of HGPIN difficult
Predominant Pattern/Injury Type
Not applicable
Adjuncts in Work-Up and Diagnosis
Ancillary IHC helpful in resolving some ASAP cases as PCa or benign glands
Prospectively obtaining intervening unstained slides is important and beneficial
Multiple levels (ideally 3) should be on each H&E slide to enhance representation of atypical focus
Deeper levels, particularly if atypical focus is present in last level, may produce more diagnostic features
When atypical glands are present in conjunction with separate cores showing definitive cancer, there may be need for further evaluation if diagnosis of PCa would upstage tumor (e.g., bilateral involvement)
Internal &/or external expert consultation may help resolve issue of diagnostic uncertainty
For some cases, ASAP remains best diagnosis
ANCILLARY TESTS
Immunohistochemistry
2 general approaches (philosophies) to interpreting adjunctive IHC in diagnosis of focal PCa
Use to establish definitive diagnosis as PCa
To confirm very strong impression of carcinoma
To confirm carcinoma of morphologically subtle subtype
Use to exclude benign process, which would preclude ASAP designation
Typically in cases with less diagnostic certainty
When partial atrophy and other mimics are considered
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree