Atypical Mycobacterial Lymphadenitis
Tariq Muzzafar, MBBS
Key Facts
Etiology/Pathogenesis
Peak incidence at 1-5 years
M. avium-intracellulare (in 80% of cases in children)
M. scrofulaceum, M. malmoense, and M. haemophilum
Uncommon in adults with exception of AIDS patients in era of HAART
Diagnosis requires excluding M. tuberculosis infection and
Positive culture for AM or
Suggestive histologic findings
Microscopic Pathology
Lymph node architecture partially or totally effaced
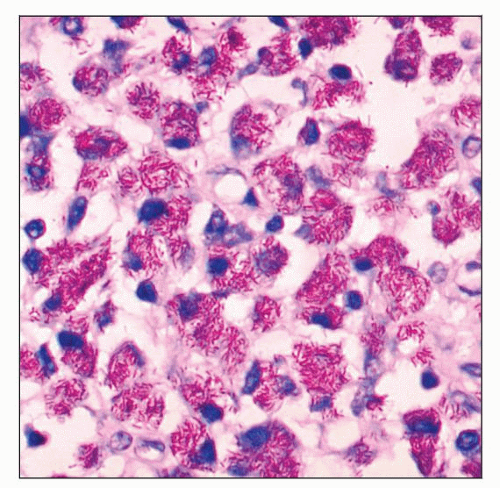
Sheets of large pale histiocytes with abundant foamy cytoplasm
Acute inflammation can be present
Rarely, multinucleated giant cells
Usually no granulomas, necrosis, calcification, fibrosis
Ancillary Tests
Acid-fast stains: Abundant AFB within histiocytes
Culture essential for definitive identification
High-performance liquid chromatography
Genotypic methods
Top Differential Diagnoses
M. tuberculosis lymphadenitis
Mycobacterial spindle cell pseudotumor
Fungal lymphadenitis
Kikuchi-Fujimoto lymphadenitis
Reporting Considerations
Not reportable in USA since these are not communicable
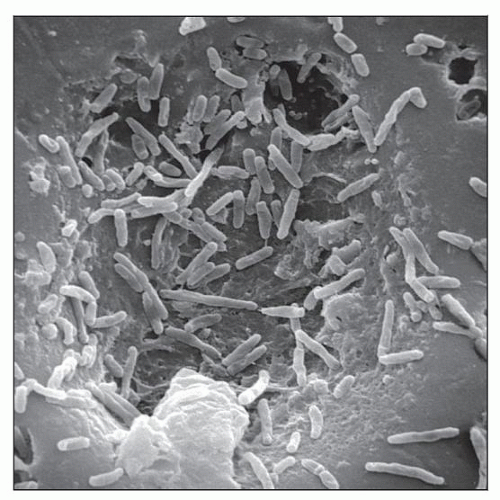 Scanning electron micrograph demonstrates M. chelonae bacteria. (Courtesy J. Carr, CDC Public Health Image Library, #226.) |
TERMINOLOGY
Abbreviations
Atypical mycobacteria (AM)
Synonyms
Nontuberculous mycobacteria (NTM)
Mycobacteria other than tubercle bacilli (MOT)
Potentially pathogenic environmental mycobacteria (PPEM)
Definitions
Lymphadenitis caused by infection by atypical mycobacteria
ETIOLOGY/PATHOGENESIS
Environmental Exposure
Widely distributed in soil; also present in natural and treated water sources
No evidence of animal-to-human or human-to-human transmission
Human infection suspected to be acquired from environmental sources
Infectious Agents
M. avium-intracellulare (MAI) is most common organism implicated in lymphadenitis
M. scrofulaceum, M. fortuitum, M. chelonei, M. abscessus, M. kansasii also occur but are less common
May cause either asymptomatic infection or symptomatic disease
Pathogenesis
Nonspecific immunity
Epithelial barrier integrity
Gastric pH
Interleukin (IL)-8, IL-12, chemokine ligand 5 (CCL5)
Natural resistance-associated macrophage protein
Macrophages initially phagocytose mycobacteria
Specific immunity
Develops over weeks following infection
Mediated by CD4(+) T lymphocytes
Involves IL-2, interferon (IFN)-γ, tumor necrosis factor (TNF)-γ
IFN-γ activates neutrophils and macrophages to kill intracellular mycobacteria
Host defects predispose to disseminated infection
Deficiency of CD4(+) lymphocytes in HIV infection
Disseminated AM infection when CD4(+) count < 50/µl
Specific mutations resulting in IFN-γ receptor defects and reduced IFN-γ production
CLINICAL ISSUES
Epidemiology
Incidence
Infection rate in North America: 1-12 per 100,000 persons
Disease rates: 0.1-2 per 100,000 persons
Prevalence of pulmonary AM infection in USA is increasing
MAI is most common AM species causing disease
Many other species have been implicated
Significant percentage of adults have had prior asymptomatic infection with AM as assessed by skin tests
Distinguishing infection from disease needs clinical correlation
Diagnostic criteria have been defined to guide treatment
Infection defined as isolation of viable organisms from uncontaminated specimen in absence of clinical manifestations
Disease defined as additional signs or symptoms that suggest pathogenic process
Isolation of a single positive sputum culture does not necessarily represent disease
No evidence that AM are associated with reactivation of disease
Prevention of infections not possible at present
Site
Virtually any body site can be infected by AM
Presentation
Pulmonary
Most common clinical manifestation
Immunocompetent patients
Tuberculosis-like pattern involving upper lobes in men with history of smoking or lung disease
Nodular bronchiectasis in slender older nonsmoking women with skeletal deformities; presents with cough
Hypersensitivity pneumonitis associated with hot tubs and medicinal baths; presents with dyspnea, cough, and fever
M. kansasii, M. xenopi, M. malmoense, and MAI implicated in tuberculosis-like pattern
MAI implicated in all nodular bronchiectasis and hypersensitivity pneumonitis patterns
Isolated pulmonary disease due to MAI occurs typically in immunocompetent adults
Immunocompromised (HIV+)
AM commonly isolated from respiratory secretions
Isolated lung disease uncommon
Extrapulmonary or disseminated disease more likely
M. kansasii can cause lung disease without dissemination
Diagnosis of pulmonary MAI based on clinical, microbiological, and radiographic criteria
Lymph nodes
Painless swelling of one or more lymph nodes in regional distribution
Anterior cervical lymph nodes most commonly affected
Submandibular, submaxillary, and preauricular
Parotid, postauricular, mediastinal lymph nodes can be involved
Peak incidence at 1-5 years of age
MAI isolated in 80% of culture-positive cases in children
Other species: M. scrofulaceum, M. malmoense, and M. haemophilum
Recently identified slow-growing mycobacteria have also been implicated
No systemic symptoms
Indolent disease
Unilateral in 95% of cases
Route of infection hypothesized to be lymphatics draining mouth and oropharynx
Lymph nodes enlarge and may rapidly soften and rupture
Chronic, draining fistulae to skin can result
Spontaneous regression can occur
Healing usually occurs by fibrosis and calcification
Uncommon in adults except AIDS patients in era of highly active antiretroviral therapy (HAART)
In past, MAI disease in AIDS patients was disseminated process
With advent of HAART, lymphadenitis can occur as part of immune reconstitution syndrome
Diagnosis of AM lymphadenitis requires either positive culture or suggestive histopathology after ruling out M. tuberculosis infection
Most patients have < 10 mm reaction due to crossreactivity between M. tuberculosis and AM proteins
Induration > 10 mm has been reported in nearly 1/3 of children with AM lymphadenitis
Skin and soft tissue
MAI infection
Occurs by direct inoculation (trauma, surgery, or injection)
Ulceration, abscess with sinus formation, erythematous plaque with crusted base ensue
Lesions are indolent
Diagnosis requires high index of suspicion
History of exposure to potential source of infection may be helpful
Combination of excision (or surgical debridement) and chemotherapy required
Buruli ulcer
M. ulcerans is causative organism
Tropical and subtropical regions: West and Central Africa, Central and South America, and Southeast Asia
Infection thought to occur through cut or wound contaminated with water, soil, or vegetation
More common in children < 15 years of age
Lower limbs involved more than upper limbs
Begins as solitary painless subcutaneous nodule or papule
Evolves to form ulcer with undermined edges
Spontaneous healing in 4-6 months
Extensive scar formation
Dissemination, including osteomyelitis, can occur, especially in patients < 15 years old
Surgical excision with wide margins required
M. marinum infection
Worldwide distribution
Infection occurs through injury by fish fins or bites, cutaneous trauma, exposure to contaminated water
Infections limited to skin and confined to single extremity
Tender, erythematous or bluish papulonodular lesion (0.5-3 cm) enlarges slowly and suppurates
Infection may spread to deeper structures, leading to scarring
Infection may extend to regional lymph nodes
Rapidly growing atypical mycobacteria (RGM)
Survive in harsh aquatic conditions; piped water systems
Resistant to sterilizing agents and disinfectants
Immunocompetent: Single lesion after penetrating trauma, surgery; M. fortuitum predominant organism
Immunocompromised: Multiple/disseminated lesions after penetrating trauma, surgery; M. chelonae or M. abscessus predominant organisms
Identification of RGM at species level essential for deciding treatment regimen
Musculoskeletal
Most affected patients are immunocompetent
Tendon sheaths, bursae, bones, and joints involved
Hand and wrist most common sites
Contiguous infection from site of surgical procedure or penetrating trauma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree