5.1 OVERVIEW OF VIRUS REPLICATION
The aim of a virus is to replicate itself, and in order to achieve this aim it needs to enter a host cell, make copies of itself, and get the new copies out of the cell. In general the process of virus replication can be broken down into seven steps:
The first letter from each step gives the abbreviation AETTGAE, which may provide a memory aid.
When trying to understand the modes of replication of different types of virus these steps provide a useful template. It is, however, a general template, because not all of the seven steps are relevant to all viruses, the steps do not always occur in the same order, and some viruses have an additional step! In the later stages of replication several steps occur concurrently. For many viruses, transcription, translation, genome replication, virion assembly, and exit can all be in progress at the same time.
In this chapter we discuss the first two steps of this generalized replication cycle: attachment and entry into the host cell. We concentrate mainly on the mechanisms used by animal viruses to gain entry into host cells, but bacteriophages are also considered. The following three chapters deal with the remaining five steps of the generalized replication cycle.
The first step, attachment of a virion to a cell, applies to viruses that infect animal and bacterial hosts. Before these viruses can cross the outer membrane or wall of the host cell they must first bind to specific molecules on the cell surface. Most plant viruses, on the other hand, are delivered directly into a cell by a vector (Section 4.2).
5.2 ANIMAL VIRUSES
5.2.1 Cell receptors and co-receptors
A virion attaches via one or more of its surface proteins to specific molecules on the surface of a host cell. These cellular molecules are known as receptors and the recognition of a receptor by a virion is highly specific, like a key fitting in its lock. It has been found that some viruses need to bind to a second type of cell surface molecule (a co-receptor) in order to infect a cell. In at least some cases, binding to a receptor causes a conformational change in the virus protein that enables it to bind to the co-receptor.
Receptors and co-receptors are cell surface molecules with a wide range of functions that include:
- acting as receptors for chemokines and growth factors;
- mediating cell-to-cell contact and adhesion.
Some cell surface molecules used by viruses as receptors are sugars, but most are glycoproteins; some examples are given in Table 5.1.
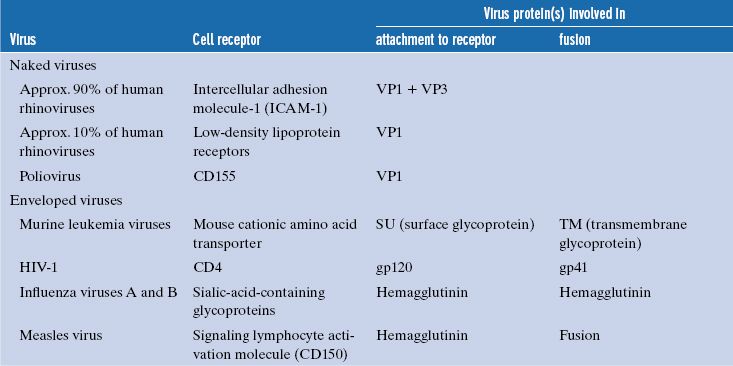
Table 5.1 Examples of cell receptors, virus proteins involved in attachment, and (for enveloped viruses) fusion proteins
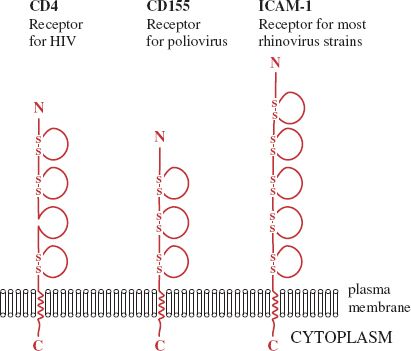
A number of receptors are glycoproteins that are folded into domains similar to those found in immunoglobulin molecules. Three examples are shown in Figure 5.1; in each molecule the binding site for the virus is located in the outermost domain.
Figure 5.1 Cell receptors with immunoglobulin-like domains. Each of these molecules is a glycoprotein; the sugar side-chains are not shown. Each loop indicates an immunoglobulin-like domain. Most of the domains are stabilized by one or two disulfide (—S—S—) bonds. In each receptor the site that the virus binds to is located in the outer domain.
Many of the cell surface molecules used by viruses as receptors are in regions of the plasma membrane that are coated on the inner surface with one of the proteins clathrin or caveolin.
5.2.1.a Evidence that a cell surface molecule is a virus receptor
A search for the receptor for a particular virus might begin with the production of a panel of mono-clonal antibodies against cell surface proteins. If one of the antibodies blocks virus binding and infectivity then this is strong evidence that the corresponding antigen is the receptor.
Further evidence that a molecule is a virus receptor might come from experiments that show one or more of the following:
- soluble derivatives of the molecule block virus binding/infectivity;
- the normal ligand for the molecule blocks virus binding/infectivity;
- introduction of the gene encoding the molecule into virus-resistant cells, and expression of that gene, makes those cells susceptible to infection.
5.2.2 Virus attachment sites
Each virion has multiple sites that can bind to receptors, and each site is made up of regions of one or more protein molecules. Some examples of these proteins are given in Table 5.1. The attachment sites of naked viruses are on the capsid surface, sometimes within depressions (e.g. poliovirus), and sometimes on ridges (e.g. foot and mouth disease virus). Poliovirus and foot and mouth disease virus are picornaviruses, and it has been demonstrated for picornaviruses in general that binding to receptors induces major structural changes in the virion (Section 14.4.1).
The attachment sites of some naked viruses are on specialized structures, such as the fibers and knobs of adenoviruses (Section 3.4.2.c) and the spikes of rotaviruses (Chapter 13), while the attachment sites of enveloped viruses are on glycoproteins in the envelope.
Some virion surface proteins that bear the attachment sites are able to bind strongly to red blood cells of various species and cause them to clump, a phenomenon known as hemagglutination. The proteins responsible for hemagglutination are called hemagglutinins. Examples of viruses that can hemagglutinate are influenza viruses (Figure 16.1) and measles virus.
5.2.3 Attachment of virions to receptors
The forces that bind virions to receptors include hydrogen bonds, ionic attractions, and van der Waals forces. Sugar moieties on the receptor and/or on the virion are commonly involved in these forces. No covalent bonds are formed between virions and receptors.
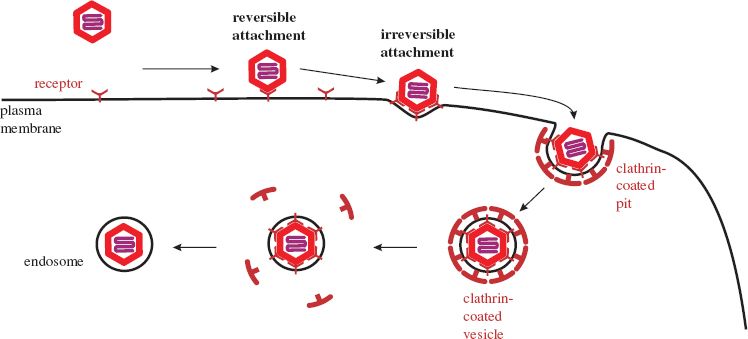
Initially, a virion is weakly bound to a cell at only one or a few receptors. At this stage the attachment is reversible and the virion may detach, but if it remains attached there are opportunities for more receptors to bind, and if sufficient bind then the attachment to the cell becomes irreversible.
5.2.4 Entry of animal viruses into cells
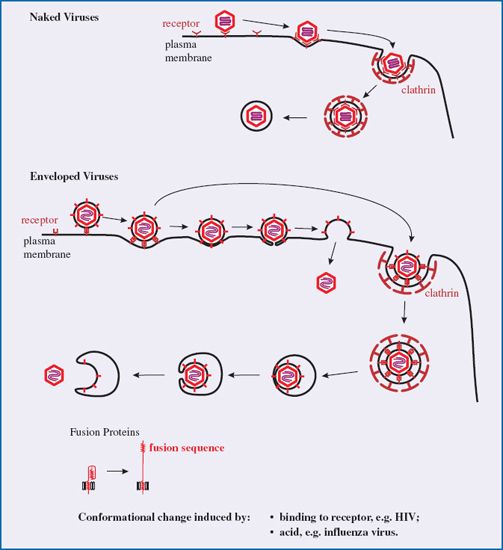
After binding to receptors animal viruses must cross the plasma membrane to gain entry to the host cell. They may do this either at the cell surface or they may cross the membrane of an endosome, which is a vesicle formed by part of the plasma membrane pinching off into the cytoplasm. This process (endocytosis) is used by cells for a variety of functions, including nutrient uptake and defense against pathogens. There are a number of endocytic mechanisms, including clathrin-mediated endocytosis and caveolin-mediated endocytosis; clathrin and caveolin are cell proteins. Most animal viruses hijack one or more of these mechanisms in order to gain access to their host cells.
Many viruses, such as adenoviruses and vesicular stomatitis virus, are taken into the host cell by clathrin-mediated endocytosis. Molecules of clathrin accumulate on the inner side of the plasma membrane at the site where the virion has attached. The clathrin forces the membrane to bend around the virion, forming a pit, which is pinched off to form a clathrin-coated vesicle (Figure 5.2). The clathrin is lost from the vesicle leaving the virion in an endosome. Some viruses, such as simian virus 40, are endocytosed at caveolin-coated regions of the plasma membrane and the virions end up in caveolin-coated endosomes. Other viruses are taken up by mechanisms that are independent of clathrin and caveolin.
Figure 5.2 Attachment and entry of a naked virion. As more receptors bind to the virion its attachment to the cell becomes irreversible. The process shown here is clathrin-mediated endocytosis. The membrane of the endosome is formed by pinching off from the plasma membrane.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


