I. Symbols Used in Respiratory Physiology
| Print
| P | Partial pressure of a gas (mm Hg) |
| V | Volume of a gas (mL) |
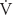 | Flow of gas (mL/min, L/s) |
| Q | Volume of blood (mL) |
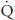 | Blood flow (mL/min) |
| F | Fractional concentration of a gas |
| C | Content or concentration of a substance in the blood (milliliters per 100 mL of blood) |
| S | Saturation in the blood (%) |
| I | Inspired |
| E | Expired |
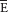 | Mixed expired |
| A | Alveolar |
| T | Tidal |
| D | Dead space |
| a | Arterial |
| v | Venous |
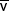 | Mixed venous |
| c | Capillary |
| c′ | End capillary |
II. The Laws Governing the Behavior of Gases
1. Avogadro’s hypothesis Equal volumes of different gases at equal temperatures contain the same number of molecules. Similarly, equal numbers of molecules in identical volumes and at the same temperature will exert the same pressure. (One mole of any gas will contain 6.02 × 1023 molecules and will occupy a volume of 22.4 L at a temperature of 0°C and a pressure of 760 mm Hg.)
2. Dalton’s law In a gas mixture the pressure exerted by each individual gas in a space is independent of the pressures of other gases in the mixture, for example,



