Apocrine Carcinoma
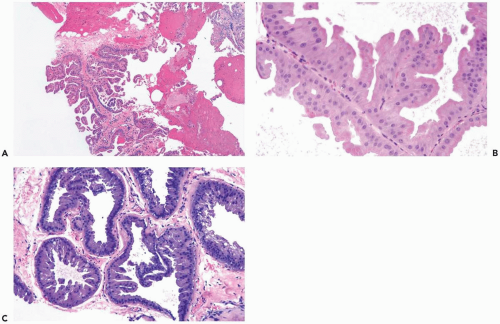
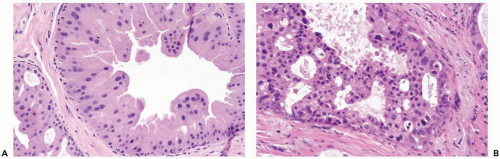
Some apocrine carcinomas probably arise from preexisting apocrine metaplasia (Fig. 16.1). In other cases, apocrine traits appear to be an intrinsic property of the carcinoma. Apocrine metaplasia is particularly abundant and often atypical in the breasts of women with apocrine carcinoma (Fig. 16.2). Transitions from atypical hyperplastic apocrine lesions to carcinoma are evident in some but not all examples of apocrine carcinoma (1). A review of the pathology and clinical aspects of apocrine breast lesions was published by O’Malley and Bane (2). The clinical significance of the “loss of heterozygosity and allelic imbalance” in some examples of apocrine metaplasia remains to be determined (3).
The diagnosis of apocrine carcinoma should be reserved for neoplasms in which all or nearly all of the epithelium has apocrine cytologic features. One percent to 2% of breast carcinomas qualify as true apocrine carcinomas. Apocrine carcinomas are usually classified as ductal. An apocrine variant of lobular carcinoma has been described, but these reports predate the availability of the E-cadherin immunostain (4,5). It has recently been observed that E-cadherin-positive apocrine intraductal carcinoma can involve the epithelium of ducts and lobules with a pagetoid distribution that mimics lobular carcinoma in situ.
No specific clinical or mammographic features associated with apocrine duct carcinomas are present (6). The reported age at diagnosis ranges from ages 19 to 86, with a distribution not significantly different from patients with nonapocrine duct carcinoma. In rare instances, the mammogram in a patient with extensive intraductal apocrine carcinoma displays diffuse “mixed form” linear and punctate calcifications “characterized by a strikingly wild, chaotic appearance with profuse deposition of calcium” (7). Patients who have invasive apocrine carcinoma usually present with a mass. The frequency of bilaterality in patients with apocrine carcinoma in one breast is not exceptional. Apocrine carcinoma of the male breast is very uncommon. One unusual apocrine male mammary carcinoma had a glandular structure and psammoma bodies (8). When studied by immunohistochemistry, 98% of 102 apocrine lesions, including benign conditions and carcinomas, were ER- and PR-negative (9). In the latter series, androgen receptors were present in 94% of benign lesions and 72% of carcinomas with apocrine differentiation. Apocrine carcinomas, which are ER-negative by immunohistochemistry, may express estrogen receptor mRNA (10).
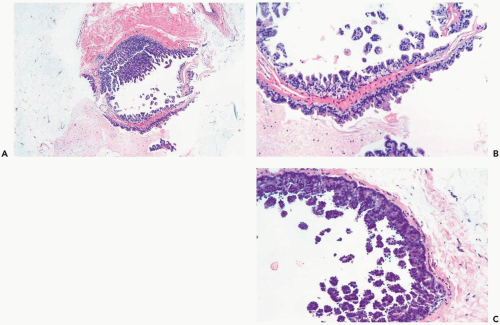
The distinction between atypical apocrine hyperplasia and apocrine intraductal carcinoma is sometimes difficult (Fig. 16.3). Carter and Rosen (11) described sclerosing breast lesions with atypical apocrine epithelium characterized by nuclear atypia, varying degrees of cytoplasmic clearing, and rare mitoses. A critical feature in the distiction between atypical hyperplasia and apocrine intraductal carcinoma in a sclerosing lesion is the extent of epithelial expansion. In situ apocrine carcinoma is diagnosed only when there is enough neoplastic epithelial proliferation to produce one of the characteristic growth patterns of intraductal carcinoma (Fig. 16.4).
The characterization of atypical apocrine lesions on the basis of size as well as cytologic and structural criteria has been suggested. For example, Tavassoli and Norris (12) considered apocrine ductal lesions that occupied an area of less than 2 mm to be atypical apocrine hyperplasia, regardless of cytologic and structural features. Larger histologically identical foci qualified as apocrine intraductal carcinoma. O’Malley et al. (13) used a combination of cytologic criteria and lesional diameter to define a “borderline” group of apocrine lesions. Foci with “borderline” cytologic features were considered to be apocrine intraductal carcinoma if larger than 8 mm. “Borderline” or atypical apocrine hyperplasias were proliferative foci smaller than 8 mm with nuclear atypia lacking the characteristic irregular nuclear membranes, coarse chromatin, and large, often multiple nucleoli of apocrine carcinoma.
The diagnosis of apocrine lesions on the basis of size is not readily applicable to needle core biopsy specimens. The precision of the requisite measurements is highly unreliable and subject to many uncontrolled variables. A cluster of closely connected duct cross sections with little intervening stroma could occupy an area less than 2 mm, leading to a diagnosis of atypical apocrine hyperplasia on the basis of the 2-mm “rule,” whereas the same group of duct sections separated by more stroma would encompass an area greater than 2 mm and qualify as intraductal carcinoma. Because these microscopic lesions are rarely
appreciated grossly, there is no assurance that the tissue is oriented in a paraffin block so that the plane of section represents the maximum diameter. These issues are further compounded in a needle core biopsy specimen if the lesion is seen in more than one tissue core because the spatial relationship of the pieces is indeterminate. Consequently, these so-called criteria cannot be accepted as guidelines for the interpretation of surgical or needle core biopsy specimens.
appreciated grossly, there is no assurance that the tissue is oriented in a paraffin block so that the plane of section represents the maximum diameter. These issues are further compounded in a needle core biopsy specimen if the lesion is seen in more than one tissue core because the spatial relationship of the pieces is indeterminate. Consequently, these so-called criteria cannot be accepted as guidelines for the interpretation of surgical or needle core biopsy specimens.
Cytologic features of in situ and invasive apocrine carcinomas are manifested in the nuclei and in the cytoplasm. The nuclei are enlarged and pleomorphic when compared to the nuclei of benign apocrine cells. They usually contain prominent and eosinophilic or basophilic nucleoli (Figs. 16.2, 16.5




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree