33e | Video Library of Gait Disorders |
Problems with gait and balance are major causes of falls, accidents, and resulting disability, especially in later life, and are often harbingers of neurologic disease. Early diagnosis is essential, especially for treatable conditions, because it may permit the institution of prophylactic measures to prevent dangerous falls and also to reverse or ameliorate the underlying cause. In this video, examples of gait disorders due to Parkinson’s disease, other extrapyramidal disorders, and ataxias, as well as other common gait disorders, are presented.
34 | Confusion and Delirium |
Confusion, a mental and behavioral state of reduced comprehension, coherence, and capacity to reason, is one of the most common problems encountered in medicine, accounting for a large number of emergency department visits, hospital admissions, and inpatient consultations. Delirium, a term used to describe an acute confusional state, remains a major cause of morbidity and mortality, costing over $150 billion dollars yearly in health care costs in the United States alone. Despite increased efforts targeting awareness of this condition, delirium often goes unrecognized in the face of evidence that it is usually the cognitive manifestation of serious underlying medical or neurologic illness.
CLINICAL FEATURES OF DELIRIUM
A multitude of terms are used to describe patients with delirium, including encephalopathy, acute brain failure, acute confusional state, and postoperative or intensive care unit (ICU) psychosis. Delirium has many clinical manifestations, but is defined as a relatively acute decline in cognition that fluctuates over hours or days. The hallmark of delirium is a deficit of attention, although all cognitive domains—including memory, executive function, visuospatial tasks, and language—are variably involved. Associated symptoms that may be present in some cases include altered sleep-wake cycles, perceptual disturbances such as hallucinations or delusions, affect changes, and autonomic findings that include heart rate and blood pressure instability.
Delirium is a clinical diagnosis that is made only at the bedside. Two subtypes have been described—hyperactive and hypoactive—based on differential psychomotor features. The cognitive syndrome associated with severe alcohol withdrawal (i.e., “delirium tremens”) remains the classic example of the hyperactive subtype, featuring prominent hallucinations, agitation, and hyperarousal, often accompanied by life-threatening autonomic instability. In striking contrast is the hypoactive subtype, exemplified by benzodiazepine intoxication, in which patients are withdrawn and quiet, with prominent apathy and psychomotor slowing.
This dichotomy between subtypes of delirium is a useful construct, but patients often fall somewhere along a spectrum between the hyperactive and hypoactive extremes, sometimes fluctuating from one to the other. Therefore, clinicians must recognize this broad range of presentations of delirium to identify all patients with this potentially reversible cognitive disturbance. Hyperactive patients are often easily recognized by their characteristic severe agitation, tremor, hallucinations, and autonomic instability. Patients who are quietly hypoactive are more often overlooked on the medical wards and in the ICU.
The reversibility of delirium is emphasized because many etiologies, such as systemic infection and medication effects, can be treated easily. The long-term cognitive effects of delirium remain largely unknown. Some episodes of delirium continue for weeks, months, or even years. The persistence of delirium in some patients and its high recurrence rate may be due to inadequate initial treatment of the underlying etiology. In other instances, delirium appears to cause permanent neuronal damage and cognitive decline. Even if an episode of delirium completely resolves, there may be lingering effects of the disorder; a patient’s recall of events after delirium varies widely, ranging from complete amnesia to repeated re-experiencing of the frightening period of confusion, similar to what is seen in patients with posttraumatic stress disorder.
RISK FACTORS
An effective primary prevention strategy for delirium begins with identification of patients at high risk for this disorder, including those preparing for elective surgery or being admitted to the hospital. Although no single validated scoring system has been widely accepted as a screen for asymptomatic patients, there are multiple well-established risk factors for delirium.
The two most consistently identified risks are older age and baseline cognitive dysfunction. Individuals who are over age 65 or exhibit low scores on standardized tests of cognition develop delirium upon hospitalization at a rate approaching 50%. Whether age and baseline cognitive dysfunction are truly independent risk factors is uncertain. Other predisposing factors include sensory deprivation, such as preexisting hearing and visual impairment, as well as indices for poor overall health, including baseline immobility, malnutrition, and underlying medical or neurologic illness.
In-hospital risks for delirium include the use of bladder catheterization, physical restraints, sleep and sensory deprivation, and the addition of three or more new medications. Avoiding such risks remains a key component of delirium prevention as well as treatment. Surgical and anesthetic risk factors for the development of postoperative delirium include specific procedures such as those involving cardiopulmonary bypass, inadequate or excessive treatment of pain in the immediate postoperative period, and perhaps specific agents such as inhalational anesthetics.
The relationship between delirium and dementia (Chap. 448) is complicated by significant overlap between the two conditions, and it is not always simple to distinguish between them. Dementia and preexisting cognitive dysfunction serve as major risk factors for delirium, and at least two-thirds of cases of delirium occur in patients with coexisting underlying dementia. A form of dementia with parkinsonism, termed dementia with Lewy bodies, is characterized by a fluctuating course, prominent visual hallucinations, parkinsonism, and an attentional deficit that clinically resembles hyperactive delirium; patients with this condition are particularly vulnerable to delirium. Delirium in the elderly often reflects an insult to the brain that is vulnerable due to an underlying neurodegenerative condition. Therefore, the development of delirium sometimes heralds the onset of a previously unrecognized brain disorder.
EPIDEMIOLOGY
Delirium is common, but its reported incidence has varied widely with the criteria used to define this disorder. Estimates of delirium in hospitalized patients range from 18 to 64%, with higher rates reported for elderly patients and patients undergoing hip surgery. Older patients in the ICU have especially high rates of delirium that approach 75%. The condition is not recognized in up to one-third of delirious inpatients, and the diagnosis is especially problematic in the ICU environment, where cognitive dysfunction is often difficult to appreciate in the setting of serious systemic illness and sedation. Delirium in the ICU should be viewed as an important manifestation of organ dysfunction not unlike liver, kidney, or heart failure. Outside the acute hospital setting, delirium occurs in nearly one-quarter of patients in nursing homes and in 50 to 80% of those at the end of life. These estimates emphasize the remarkably high frequency of this cognitive syndrome in older patients, a population expected to grow in the upcoming decades.
Until recently, an episode of delirium was viewed as a transient condition that carried a benign prognosis. It is now recognized as a disorder with a substantial morbidity rate and increased mortality rate and often represents the first manifestation of a serious underlying illness. Recent estimates of in-hospital mortality rates among delirious patients have ranged from 25 to 33%, a rate similar to that of patients with sepsis. Patients with an in-hospital episode of delirium have a fivefold higher mortality rate in the months after their illness compared with age-matched nondelirious hospitalized patients. Delirious hospitalized patients have a longer length of stay, are more likely to be discharged to a nursing home, and are more likely to experience subsequent episodes of delirium and cognitive decline; as a result, this condition has enormous economic implications.
PATHOGENESIS
The pathogenesis and anatomy of delirium are incompletely understood. The attentional deficit that serves as the neuropsychological hallmark of delirium has a diffuse localization within the brainstem, thalamus, prefrontal cortex, and parietal lobes. Rarely, focal lesions such as ischemic strokes have led to delirium in otherwise healthy persons; right parietal and medial dorsal thalamic lesions have been reported most commonly, pointing to the importance of these areas to delirium pathogenesis. In most cases, delirium results from widespread disturbances in cortical and subcortical regions rather than a focal neuroanatomic cause. Electroencephalogram (EEG) data in persons with delirium usually show symmetric slowing, a nonspecific finding that supports diffuse cerebral dysfunction.
Multiple neurotransmitter abnormalities, proinflammatory factors, and specific genes likely play a role in the pathogenesis of delirium. Deficiency of acetylcholine may play a key role, and medications with anticholinergic properties also can precipitate delirium. Dementia patients are susceptible to episodes of delirium, and those with Alzheimer’s pathology and dementia with Lewy bodies or Parkinson’s disease dementia are known to have a chronic cholinergic deficiency state due to degeneration of acetylcholine-producing neurons in the basal forebrain. Additionally, other neurotransmitters are also likely to be involved in this diffuse cerebral disorder. For example, increases in dopamine can also lead to delirium. Patients with Parkinson’s disease treated with dopaminergic medications can develop a delirium-like state that features visual hallucinations, fluctuations, and confusion.
Not all individuals exposed to the same insult will develop signs of delirium. A low dose of an anticholinergic medication may have no cognitive effects on a healthy young adult but produce a florid delirium in an elderly person with known underlying dementia, although even healthy young persons develop delirium with very high doses of anticholinergic medications. This concept of delirium developing as the result of an insult in predisposed individuals is currently the most widely accepted pathogenic construct. Therefore, if a previously healthy individual with no known history of cognitive illness develops delirium in the setting of a relatively minor insult such as elective surgery or hospitalization, an unrecognized underlying neurologic illness such as a neurodegenerative disease, multiple previous strokes, or another diffuse cerebral cause should be considered. In this context, delirium can be viewed as a “stress test for the brain” whereby exposure to known inciting factors such as systemic infection and offending drugs can unmask a decreased cerebral reserve and herald a serious underlying and potentially treatable illness.
PREVENTION
In light of the high morbidity associated with delirium and the tremendously increased health care costs that accompany it, development of an effective strategy to prevent delirium in hospitalized patients is extremely important. Successful identification of high-risk patients is the first step, followed by initiation of appropriate interventions. Simple standardized protocols used to manage risk factors for delirium, including sleep-wake cycle reversal, immobility, visual impairment, hearing impairment, sleep deprivation, and dehydration, have been shown to be effective. Recent trials in the ICU have focused both on identifying sedatives, such as dexmedetomidine, that are less likely to lead to delirium in critically ill patients and on developing protocols for daily awakenings in which infusions of sedative medications are interrupted and the patient is reorientated by the staff. All hospitals and health care systems should work toward decreasing the incidence of delirium.
35 | Dementia |
Dementia, a syndrome with many causes, affects >5 million people in the United States and results in a total annual health care cost between $157 and $215 billion. Dementia is defined as an acquired deterioration in cognitive abilities that impairs the successful performance of activities of daily living. Episodic memory, the ability to recall events specific in time and place, is the cognitive function most commonly lost; 10% of persons age >70 years and 20–40% of individuals age >85 years have clinically identifiable memory loss. In addition to memory, dementia may erode other mental faculties, including language, visuospatial, praxis, calculation, judgment, and problem-solving abilities. Neuropsychiatric and social deficits also arise in many dementia syndromes, manifesting as depression, apathy, anxiety, hallucinations, delusions, agitation, insomnia, sleep disturbances, compulsions, or disinhibition. The clinical course may be slowly progressive, as in Alzheimer’s disease (AD); static, as in anoxic encephalopathy; or may fluctuate from day to day or minute to minute, as in dementia with Lewy bodies. Most patients with AD, the most prevalent form of dementia, begin with episodic memory impairment, although in other dementias, such as frontotemporal dementia, memory loss is not typically a presenting feature. Focal cerebral disorders are discussed in Chap. 36 and illustrated in a video library in Chap. 37e; the pathogenesis of AD and related disorders is discussed in Chap. 448.
FUNCTIONAL ANATOMY OF THE DEMENTIAS
Dementia syndromes result from the disruption of specific large-scale neuronal networks; the location and severity of synaptic and neuronal loss combine to produce the clinical features (Chap. 36). Behavior, mood, and attention are modulated by ascending noradrenergic, serotonergic, and dopaminergic pathways, whereas cholinergic signaling is critical for attention and memory functions. The dementias differ in the relative neurotransmitter deficit profiles; accordingly, accurate diagnosis guides effective pharmacologic therapy.
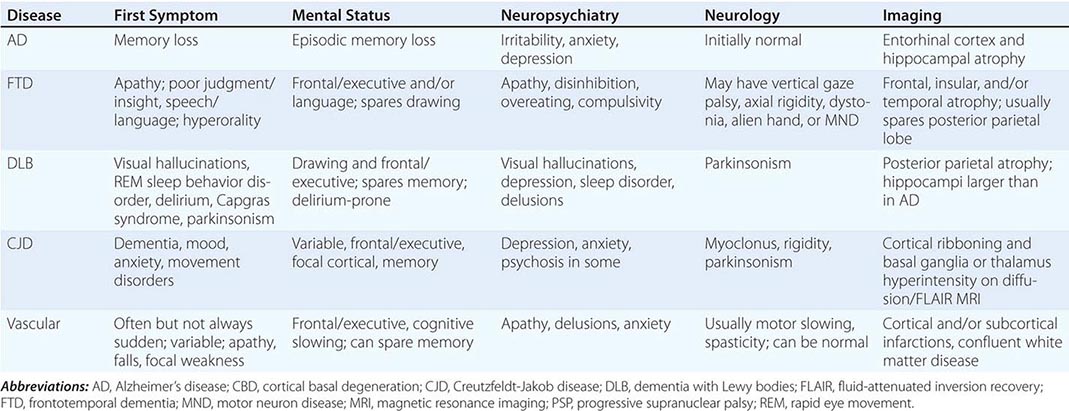
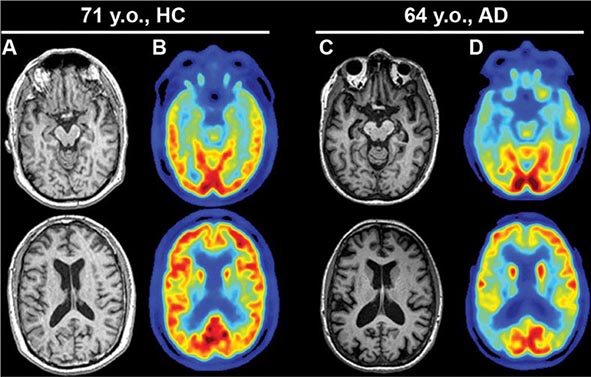
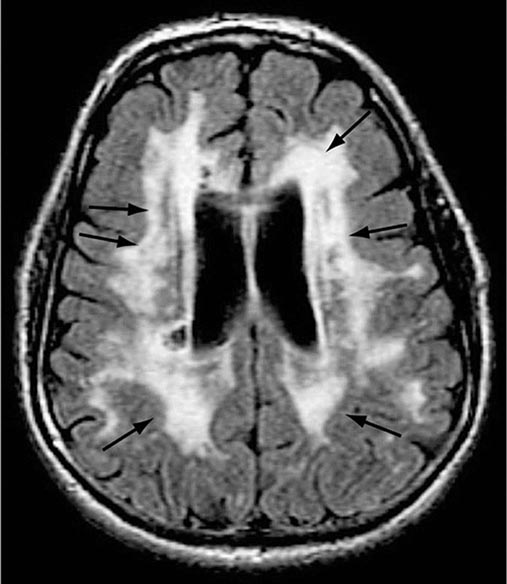
AD begins in the entorhinal region of the medial temporal lobe, spreads to the hippocampus, and then moves to lateral and posterior temporal and parietal neocortex, eventually causing a more widespread degeneration. Vascular dementia is associated with focal damage in a variable patchwork of cortical and subcortical regions or white matter tracts that disconnect nodes within distributed networks. In keeping with its anatomy, AD typically presents with episodic memory loss accompanied later by aphasia or navigational problems. In contrast, dementias that begin in frontal or subcortical regions, such as frontotemporal dementia (FTD) or Huntington’s disease (HD), are less likely to begin with memory problems and more likely to present with difficulties with judgment, mood, executive control, movement, and behavior.
Lesions of frontal-striatal1 pathways produce specific and predictable effects on behavior. The dorsolateral prefrontal cortex has connections with a central band of the caudate nucleus. Lesions of either the caudate or dorsolateral prefrontal cortex, or their connecting white matter pathways, may result in executive dysfunction, manifesting as poor organization and planning, decreased cognitive flexibility, and impaired working memory. The lateral orbital frontal cortex connects with the ventromedial caudate, and lesions of this system cause impulsiveness, distractibility, and disinhibition. The anterior cingulate cortex and adjacent medial prefrontal cortex project to the nucleus accumbens, and interruption of this system produces apathy, poverty of speech, emotional blunting, or even akinetic mutism. All corticostriatal systems also include topographically organized projections through the globus pallidus and thalamus, and damage to these nodes can likewise reproduce the clinical syndrome of cortical or striatal injury.
THE CAUSES OF DEMENTIA
The single strongest risk factor for dementia is increasing age. The prevalence of disabling memory loss increases with each decade over age 50 and is usually associated with the microscopic changes of AD at autopsy. Yet some centenarians have intact memory function and no evidence of clinically significant dementia. Whether dementia is an inevitable consequence of normal human aging remains controversial.
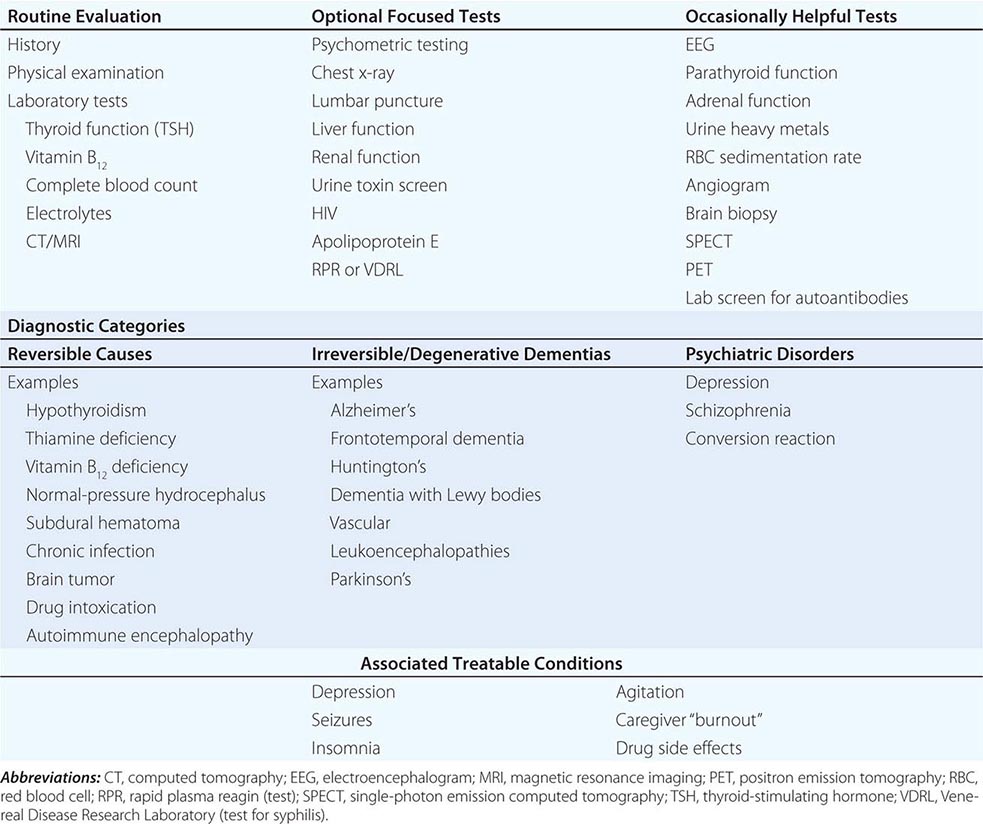
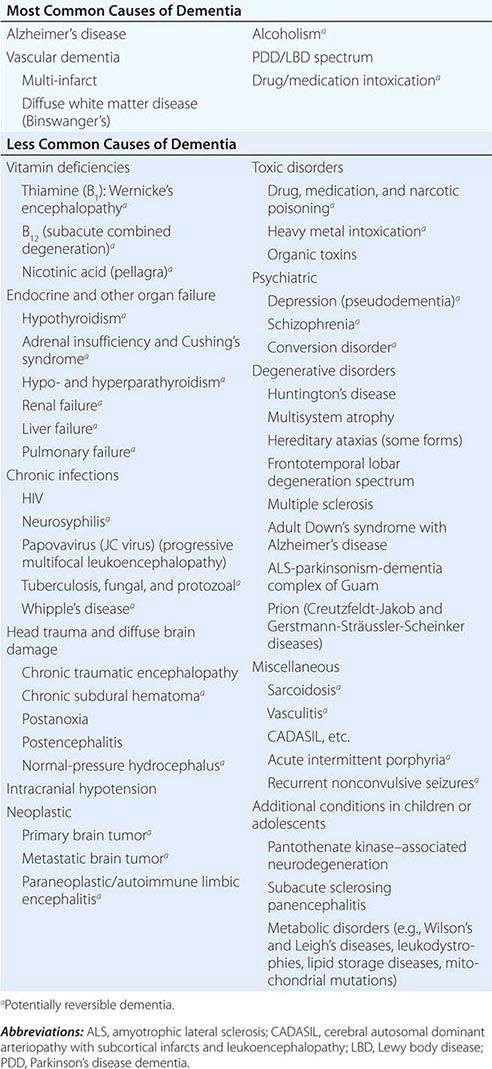
The many causes of dementia are listed in Table 35-1. The frequency of each condition depends on the age group under study, access of the group to medical care, country of origin, and perhaps racial or ethnic background. AD is the most common cause of dementia in Western countries, accounting for more than half of all patients. Vascular disease is considered the second most frequent cause for dementia and is particularly common in elderly patients or populations with limited access to medical care, where vascular risk factors are undertreated. Often, vascular brain injury is mixed with neurodegenerative disorders, making it difficult, even for the neuropathologist, to estimate the contribution of cerebrovascular disease to the cognitive disorder in an individual patient. Dementias associated with Parkinson’s disease (PD) (Chap. 449) are common and may develop years after onset of a parkinsonian disorder, as seen with PD-related dementia (PDD), or can occur concurrently with or preceding the motor syndrome, as in dementia with Lewy bodies (DLB). In patients under the age of 65, FTD rivals AD as the most common cause of dementia. Chronic intoxications, including those resulting from alcohol and prescription drugs, are an important and often treatable cause of dementia. Other disorders listed in Table 35-1 are uncommon but important because many are reversible. The classification of dementing illnesses into reversible and irreversible disorders is a useful approach to differential diagnosis. When effective treatments for the neurodegenerative conditions emerge, this dichotomy will become obsolete.
DIFFERENTIAL DIAGNOSIS OF DEMENTIA |
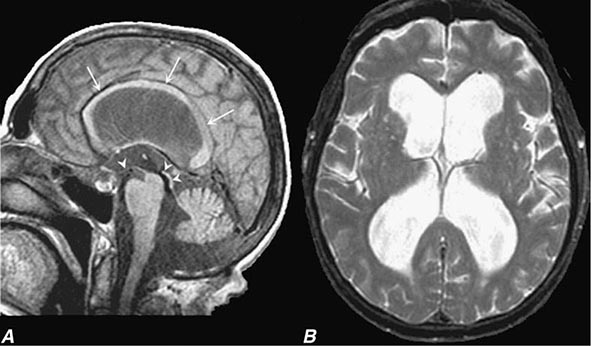
In a study of 1000 persons attending a memory disorders clinic, 19% had a potentially reversible cause of the cognitive impairment and 23% had a potentially reversible concomitant condition that may have contributed to the patient’s impairment. The three most common potentially reversible diagnoses were depression, normal pressure hydrocephalus (NPH), and alcohol dependence; medication side effects are also common and should be considered in every patient (Table 35-1).
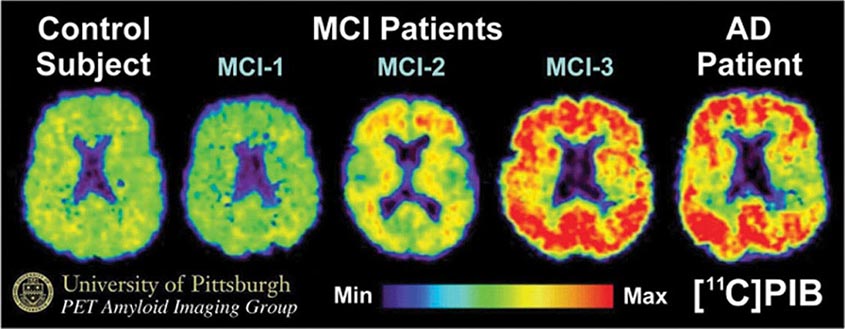
Subtle cumulative decline in episodic memory is a common part of aging. This frustrating experience, often the source of jokes and humor, is referred to as benign forgetfulness of the elderly. Benign means that it is not so progressive or serious that it impairs reasonably successful and productive daily functioning, although the distinction between benign and more significant memory loss can be difficult to make. At age 85, the average person is able to learn and recall approximately one-half of the items (e.g., words on a list) that he or she could at age 18. A measurable cognitive problem that does not seriously disrupt daily activities is often referred to as mild cognitive impairment (MCI). Factors that predict progression from MCI to an AD dementia include a prominent memory deficit, family history of dementia, presence of an apolipoprotein ε4 (Apo ε4) allele, small hippocampal volumes, an AD-like signature of cortical atrophy, low cerebrospinal fluid Aβ, and elevated tau or evidence of brain amyloid deposition on positron emission tomography (PET) imaging.
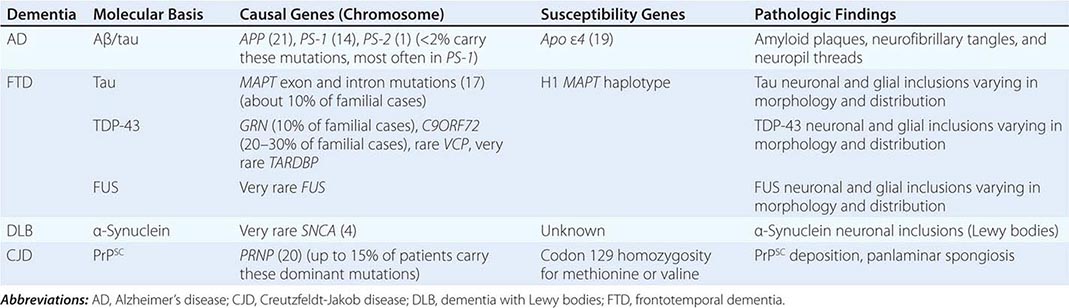
The major degenerative dementias include AD, DLB, FTD and related disorders, HD, and prion diseases, including Creutzfeldt-Jakob disease (CJD). These disorders are all associated with the abnormal aggregation of a specific protein: Aβ42 and tau in AD; α-synuclein in DLB; tau, TAR DNA-binding protein of 43 kDa (TDP-43), or fused in sarcoma (FUS) in FTD; huntingtin in HD; and misfolded prion protein (PrPsc) in CJD (Table 35-2).
THE MOLECULAR BASIS FOR DEGENERATIVE DEMENTIA |
1The striatum comprises the caudate/putamen.
36 | Aphasia, Memory Loss, and Other Focal Cerebral Disorders |
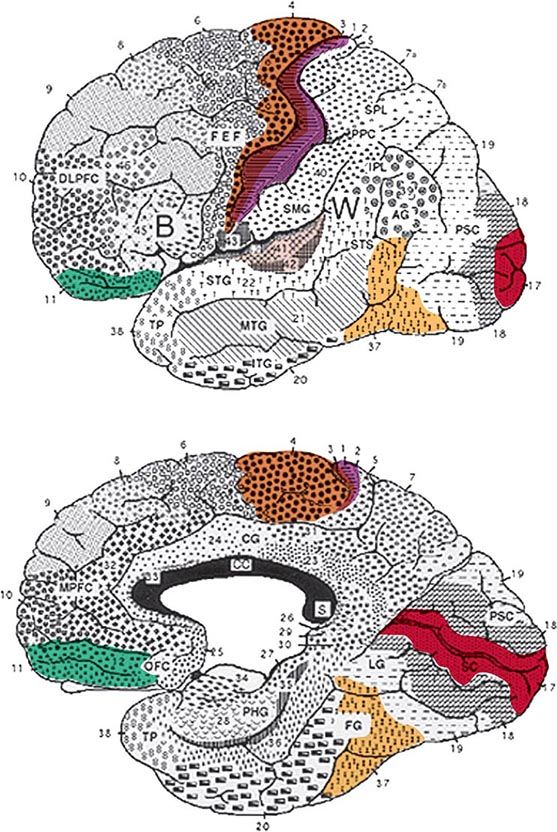
The cerebral cortex of the human brain contains approximately 20 billion neurons spread over an area of 2.5 m2. The primary sensory and motor areas constitute 10% of the cerebral cortex. The rest is subsumed by modality-selective, heteromodal, paralimbic, and limbic areas collectively known as the association cortex (Fig. 36-1). The association cortex mediates the integrative processes that subserve cognition, emotion, and comportment. A systematic testing of these mental functions is necessary for the effective clinical assessment of the association cortex and its diseases. According to current thinking, there are no centers for “hearing words,” “perceiving space,” or “storing memories.” Cognitive and behavioral functions (domains) are coordinated by intersecting large-scale neural networks that contain interconnected cortical and subcortical components. Five anatomically defined large-scale networks are most relevant to clinical practice: (1) a perisylvian network for language, (2) a parietofrontal network for spatial orientation, (3) an occipitotemporal network for face and object recognition, (4) a limbic network for retentive memory, and (5) a prefrontal network for the executive control of cognition and comportment.
FIGURE 36-1 Lateral (top) and medial (bottom) views of the cerebral hemispheres. The numbers refer to the Brodmann cytoarchitectonic designations. Area 17 corresponds to the primary visual cortex, 41–42 to the primary auditory cortex, 1–3 to the primary somatosensory cortex, and 4 to the primary motor cortex. The rest of the cerebral cortex contains association areas. AG, angular gyrus; B, Broca’s area; CC, corpus callosum; CG, cingulate gyrus; DLPFC, dorsolateral prefrontal cortex; FEF, frontal eye fields (premotor cortex); FG, fusiform gyrus; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; LG, lingual gyrus; MPFC, medial prefrontal cortex; MTG, middle temporal gyrus; OFC, orbitofrontal cortex; PHG, parahippocampal gyrus; PPC, posterior parietal cortex; PSC, peristriate cortex; SC, striate cortex; SMG, supramarginal gyrus; SPL, superior parietal lobule; STG, superior temporal gyrus; STS, superior temporal sulcus; TP, temporopolar cortex; W, Wernicke’s area.
THE LEFT PERISYLVIAN NETWORK FOR APHASIAS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree