Figure 92-1. A,B: Major visceral and parietal collateral networks that may become prominent with aortoiliac occlusive disease.

Figure 92-2. CTA with 3D reconstruction demonstrating severe aortoiliac occlusive disease with juxtarenal aortic occlusion. Note the extensive collateral networks reconstituting the inferior mesenteric artery (red arrow: marginal artery) and femoral arterial segments (blue arrow: inferior epigastric artery) (green arrow: subcostal arteries and superficial iliac circumflex artery collaterals).
Physical examination typically reveals diminished or absent femoral pulses. A bruit auscultated over the groins or lower abdomen is not specific for critical stenosis. Severely diseased, calcified femoral arteries may be palpable as firm, tubular structures in the groins. Normal femoral and distal pulses may be palpable, even in the presence of hemodynamically significant aortoiliac stenosis. These pulses, however, rapidly disappear following ambulation, as the increased flow demands of the exercising leg muscles lead to lowered peripheral vascular resistance and reduced distal arterial pressure. Patients with longstanding aortoiliac atherosclerosis may have disuse atrophy of the lower extremity musculature. Other common signs of lower extremity arterial occlusive disease include trophic changes, such as hair loss on the legs or toes, and thin, shiny skin on the feet; patients with severe reductions in pedal blood flow may display pedal rubor on limb dependency, coupled with pallor on elevation.
Critical limb ischemia (CLI) symptoms manifest in the lower leg or foot as ischemic rest pain, nonhealing wounds, or gangrenous changes. Such symptoms may be a manifestation of AIOD alone but almost always occur in combination with more distal femoropopliteal/tibial disease (i.e., multilevel arterial occlusive disease). In the absence of infrainguinal disease, aortoiliac collaterals are usually capable of maintaining adequate resting distal tissue perfusion.
Acute arterial occlusions from emboli lodging at the aortic, iliac or femoral bifurcation do not allow collateral pathways to mature and compensate for the sudden loss of blood supply. This situation leads to the acute onset of symptoms, with the resulting 5 “Ps” of acute ischemia: pain, pallor, pulselessness, paresthesia, and paralysis. Cholesterol crystal embolism, or “blue toe” syndrome, occurs when debris breaks free from an aortic, iliac, or more distal plaque, releasing platelet microthrombi, cholesterol crystals, and other atheromatous material into the arterial lumen.10 Downstream embolism into the microcirculation of the lower extremities can produce dermal discoloration in a characteristic reticular pattern (livedo reticularis), digital ischemia, or even gangrene (Fig. 92-3). Such patients usually have palpable pedal pulses. Embolic events can occur spontaneously or can be induced by interventions, such as guidewire manipulation (e.g., during angiographic procedures or placement of an intra-aortic balloon pump) or clamping an artery with unstable plaque during open vascular surgery.
Clinical Pathologic Types of Aortoiliac Disease
Different patterns of AIOD have been classically identified on preoperative imaging studies.11 Disease confined to the distal infrarenal aorta and common iliac arteries, classified as type I, accounts for only 10% of patients with inflow disease. Patients with type I disease are younger (in their 50s and 60s), more frequently female, and typically have a normal life expectancy.12,13 These patients usually present with complaints of disabling claudication in the buttocks, hips, and thighs. Such localized disease may be amenable to percutaneous transluminal angioplasty (PTA) or, as was sometimes practiced in the past, aortoiliac endarterectomy.
Type II aortoiliac disease is more extensive and more common; the atherosclerotic plaque extends distally into the external iliac arteries and may reach the common femoral bifurcations. Such patients experience worse claudication than patients with more localized disease and may present with CLI. They are usually men, present on average a decade later in life (60s and 70s), than patients with type I disease, and tend to have diabetes, hypertension, and concomitant cerebrovascular, coronary, and visceral atherosclerosis.12 As a result, these patients have a reduced life expectancy.13,14
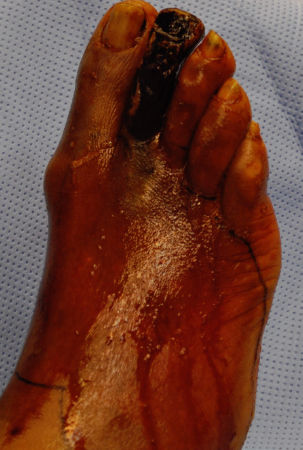
Figure 92-3. Digital atheroembolization progressed to gangrene.
Type III disease is a combination of aortoiliac and femoropopliteal and/or tibial disease. These patients with multilevel disease are usually older than those in the other two groups and present more frequently with symptoms of CLI.12
One other pattern worthy of mention is aortoiliac hypoplasia. The term small aortic syndrome or hypoplastic aortic syndrome may also be used. This pattern is encountered in young to middle-aged women who smoke cigarettes. The infrarenal aorta and iliac arteries are unusually small in caliber and hence prone to significant narrowing, even with modest disease. Operative treatment of such patients can be particularly challenging.15
In response to the rapidly evolving patterns of treatment of AIOD and the increasingly prominent role played by endovascular methods, the Trans-Atlantic Inter-Society Consensus for the Management of PAD (TASC II) has classified AIOD into four types, A through D (Table 92-1).16 The value of this classification derives from its use as a template for reporting standards and as a guideline to help direct treatment decisions. Patients with focal disease (TASC II types A and B) usually benefit from endovascular interventions, while those with more advanced disease (TASC II types C and D) are usually best managed with open surgical revascularization. However, evolving endovascular approaches are now being applied to these advanced lesions as well, with acceptable results.17 This is especially true for high-risk patients with TASC C and D disease presenting with CLI, who frequently have significant comorbidities, such as severe chronic obstructive pulmonary disease, nonreconstructible coronary artery disease, or a low cardiac ejection fraction. Although this approach may lead to less durable results than open surgical options, it is less morbid. To simplify the most current widely accepted strategy, the surgeon should apply endovascular treatment methods for type A and B patients, and lean toward open surgery for type C and D disease.
DIAGNOSIS
In most cases, diagnosis of significant AIOD and its severity can be made on the basis of history and physical examination alone. A clinical diagnosis, however, should be supplemented by noninvasive vascular testing. Such tests provide valuable information by confirming the presence of disease, assessing the degree of ischemia, objectively documenting the arterial segment(s) involved, and establishing a baseline from which the patient can be followed and appropriate interventions planned. Treatment results can be assessed by repeated testing. Such information is particularly useful in a patient with an equivocal history or physical examination. Formal imaging studies (CTA, MRA, and arteriography) are usually reserved for those patients considered for intervention.
Noninvasive Vascular Testing
Segmental Doppler-derived pressure measurements or pulse volume recordings are useful for demonstrating the physiologic significance of disease and can help localize hemodynamically significant lesions. Measurement of systolic arterial pressures at different levels of the lower extremity with a continuous wave Doppler flow probe is the simplest and most useful noninvasive method to assess arterial occlusive disease. The ratio of the ankle systolic pressure (measured at either the dorsalis pedis or the posterior tibial artery) to the brachial systolic pressure (using the higher of the two brachial pressures) is the ankle-brachial index (ABI), or pressure ratio, and is a good measure of the degree of ischemia present. Normal ABIs are generally equal to or slightly greater than 1.0 (Fig. 92-4). Peripheral arterial disease is designated when the ABI is ≤0.95. Patients with claudication usually have ABIs ranging from 0.50 to 0.90, whereas patients with rest pain, tissue loss, or gangrene have ABIs of <0.50. Some patients with claudication, as a result of excellent collaterals, may have ABI of >1.0 at rest.
Table 92-1 TASC II Classification


Figure 92-4. Segmental pressure measurements typical of normal results (A) and aortoiliac occlusive disease (B).
Determination of limb systolic pressures at different locations (with the “four-cuff” technique, where pressures are measured at the upper thigh, lower thigh, calf, and ankle) provides information concerning which arterial segments are involved with occlusive disease (Fig. 92-4) and helps define the presence of inflow artery disease, outflow (runoff artery) disease, or a combination of the two. A pressure drop of more than 20 mm Hg between adjacent levels indicates significant disease within the intervening arterial segment. A reduced upper thigh pressure signifies occlusive disease in the aortoiliac or common femoral segments.18
In patients with extensive calcification in the walls of the tibial arteries (as is frequently seen in diabetes mellitus or end-stage renal disease), the ankle pressures may not be interpretable because the vessels are too “stiff” to be properly compressed by the externally applied cuff. In this situation, the pressure in the digital arteries of the great toe can be measured, since these small vessels are generally spared calcification. A toe pressure of less than 30 mm Hg indicates severe ischemia.19 Inspection of the Doppler-derived arterial waveforms can provide additional information.
Patients with claudication occasionally have normal or nearly normal ABIs. As described previously, in such cases the arterial stenosis is not severe enough to cause a pressure drop in the limb at rest but does produce a significant hemodynamic change under conditions of higher flow rates when the distal vasculature dilates, as occurs with exercise. When the distal pressure drops because of vascular dilation, the stenosis prevents increased blood flow into the extremity so that a significant distal pressure drop results. The classic treadmill test involves walking on a treadmill at 1.5 miles per hour at a 14% incline for as long as the patient can go, up to 5 minutes. ABIs are measured before and after a treadmill exercise; a drop of 15% or more in the ABI following exercise is indicative of a hemodynamically significant occlusive disease.18
Duplex scanning of the aorta and iliac arteries has been advocated by some as a noninvasive diagnostic tool. Accurate imaging of the abdominal arteries, however, is difficult because of their deep retroperitoneal and pelvic locations, which can be further degraded by body habitus and overlying bowel gas. These problems have limited the widespread use of this modality.20 Arteriography, CTA, and MRA may be used for diagnosis, but are usually performed when interventional therapy is contemplated, and are, therefore, discussed later.
Differential Diagnosis
The diagnosis of AIOD is usually straightforward, but occasional diagnostic difficulty may arise when other causes of lower extremity pain are present. Irritation of lumbosacral nerve roots by spinal stenosis or intervertebral disc herniation may cause buttock and leg pain that is associated with activity. Such symptoms (neurogenic claudication), however, usually cannot be reproduced at the same level of activity, frequently occur when the patient is standing, and are relieved only by sitting or lying down. In addition, the pain is usually in a classic sciatic distribution.
Degenerative arthritis of the hip joints may produce similar buttock, hip, and referred thigh pain. There is usually a history of morning stiffness that progressively improves over the course of the day. Physical examination typically reveals tenderness directly over the hip joint that is exacerbated by moving the joint.
Peripheral neuropathy, particularly that associated with diabetes mellitus, may masquerade as ischemic rest pain.
In all these situations, segmental limb pressure measurements, with or without stress testing, can be helpful in determining the contribution of arterial occlusive disease to the patient’s symptoms.
TREATMENT
The aims of therapy in AIOD are to relieve symptoms and, in cases of CLI, to prevent limb loss (Algorithm 92-1). Medical therapy should be instituted in all patients with atherosclerotic disease but is insufficient as sole therapy in patients with limb-threatening ischemia.
Medical Therapy
3 Atherosclerosis is a systemic disease. Medical therapy is, therefore, key to reducing the risk associated with cardiovascular death in these patients. Risk factor modification, including treatment of diabetes mellitus, control of hypertension, and treatment of hyperlipidemia, and finally modification of lifestyle including cessation of smoking, diet, and exercise, are perhaps the most important components of the care of patients with atherosclerotic disease. Although risk factor control will not reverse the atherosclerotic process, it does limit disease progression; furthermore, some data indicate that smoking cessation lessens the severity of symptoms in many patients.21
Smoking Cessation
4 Smoking is associated with a subclinical inflammatory reaction.22 The degree of damage caused by smoking is directly related to the amount of tobacco consumed. The TASC II consensus recommends that: “All patients who are smokers or former smokers should be asked about the status of tobacco use at every visit. All patients should be strongly advised to stop smoking by their physicians. All patients should be offered pharmacotherapy, behavior modification, referral to a smoking cessation program, and counseling.”14 Smoking cessation alters the progression of the atherosclerotic process and may even reduce the severity of claudication23,24; Furthermore, it is an important step to reduce postoperative complications.25,26
Exercise
Supervised exercise programs have been consistently demonstrated to improve walking time and walking distance.27 Home and community-based therapy are effective for improving walking tolerance but are less effective than formal supervised exercise programs and are associated with a high dropout rate, underscoring the need for ongoing psychological support.22,23,28 Outcomes of supervised exercise programs have been shown to be similar and longer lasting than those of endovascular interventions for mild claudication.24,25 Patients are usually advised to walk until the pain occurs, rest until the pain subsides, and repeat the cycle, to a total of 30 minutes a day, three to five times per week. A 3- to 6-month trial of exercise should be initiated before intervention is considered in patients with intermittent claudication.
Diet
A well-balanced diet with low salt, low fat, and moderate amounts of added sugar intake, as per the American Heart Association guidelines, reduces the risk of chronic diseases in general, and cardiovascular disease in particular, and should be followed.26
Glycemic Control
The Trans-Atlantic Inter-Society Consensus (TASC II) consensus document recommends aggressive blood glucose control, with a target A1C goal of <7.0%, and as close to 6.0% as possible.16
Antiplatelet Therapy
Some form of platelet inhibition should be considered for all atherosclerotic patients in order to reduce the risk of myocardial infarction, stroke, and death.29 Aspirin is the agent of choice, but clopidogrel may be used if aspirin is not tolerated. The effectiveness of antiplatelet therapy in reducing symptoms of claudication is not yet established; however, the risk of PAD progression requiring revascularization is significantly reduced by antiplatelet therapy, compared with placebo.30 Adding aspirin to clopidogrel does not reduce major vascular events. However, the risk of major life-threatening bleeding is increased.31
Antihypertensive Therapy
Antihypertensive therapy reduces mortality from atherosclerotic cardiovascular disease. It is, therefore, recommended in the TASC II consensus document, despite the lack of data substantiating that antihypertensive therapy alters the progression of PAD.16
Lipid-Lowering Therapy
Lipid-lowering therapy with at least a moderate dose of a statin medication is recommended for all patients with atherosclerotic cardiovascular disease, irrespective of the baseline low-density lipoprotein–cholesterol. Lipid-lowering therapy reduced disease progression (as measured by arteriography), helped alleviate symptoms, and improved total walking times and pain-free walking distance.32
Other Pharmacologic Therapy
Cilostazol and naftidrofuryl can be valuable adjuncts in selected patients with severe lifestyle-limiting claudication.33,34 A therapeutic trial (3 to 6 months) may be tried. Although the mechanisms of action of these agents are unclear, a modest but significant reduction in claudication symptoms has been shown in controlled trials. Benefit has not been firmly established for other agents.
Indications for Revascularization
Revascularization is clearly indicated in patients with CLI; without intervention, these patients can progress to limb loss in a fairly short period of time. At 1 year following onset of CLI symptoms, it is estimated that 25% of patients will lose their limb, 25% will suffer a cardiovascular death, and 50% will be alive with their limbs intact.35,36 Per year, 1% to 2% of patients with claudication will progress to CLI.36
Patients with significant or repetitive atheroembolism from an aortoiliac source represent a group that clearly benefits from early operative intervention. Removal or bypass of the culprit lesion(s) eliminates the risk for further macro- and microembolism.
The treatment of patients with claudication secondary to AIOD remains somewhat controversial and must be individualized. Patients with mild to moderate symptoms can usually be treated medically with satisfactory results, while patients with severe, disabling, and lifestyle-limiting symptoms of claudication can benefit from revascularization therapy.
Preintervention Imaging Studies
Once a decision has been made to proceed with revascularization therapy, imaging studies are paramount for developing a treatment strategy.

Figure 92-5. CTA 3D reconstruction showing left iliac artery chronic total occlusion (red arrow).
Computed Tomographic Angiography
CTA with reformatting requires the intravenous administration of iodinated contrast material, followed by a timed CT scan of the pertinent anatomy. The images are then processed into coronal, sagittal, and three-dimensional image planes (Figs. 92-2, 92-5 to 92-7). For most patients, 120 mL of iodine contrast is used (300 to 370 mg of iodine per milliliter of contrast). Sensitivity and specificity are greater than 92% and 93%, respectively, in detecting >50% stenosis. The main drawbacks of CTA are the ionizing radiation and the use of iodinated contrast, which can cause contrast-induced nephropathy.37

Figure 92-6. A: CTA 3D reconstruction demonstrating right common iliac artery chronic total occlusion (red arrow) and severe left common iliac artery stenosis (blue arrow). B: Abdominal aortogram redemonstrating right common iliac artery chronic total occlusion and left common iliac artery stenosis. Note successful wire traversal of the right common iliac artery occlusion (red arrow). C: Abdominal aortogram demonstrating successful endoluminal bilateral common iliac artery revascularization following percutaneous balloon angioplasty and stent placement. Note the “kissing” stents in the distal aorta (red arrow).

Figure 92-7. A: A midline abdominal incision and bilateral groin incisions overlying the common femoral arteries offer exposure for performance of aortobifemoral bypass. B: This image demonstrates an end-to-end proximal aortic to graft reconstruction with the respective graft limbs anastomosed end to side to the common femoral arteries. C: Computed tomographic angiographic postprocessed and reformatted image demonstrating open aortobifemoral arterial reconstruction of juxtarenal aortic occlusion (see Fig. 92-2, preoperative image) with prosthetic bifurcated graft. Note endarterectomy of aorta immediately distal to renal arteries (red arrow), followed by proximal end-to-end anastomosis between graft and aorta (blue arrow). Distal anastomosis to femoral arteries is performed in end to side fashion (green arrows).
Magnetic Resonance Angiography
Contrast-enhanced MRA uses intravenous administration of paramagnetic gadolinium-based agents. With good technique, contrast-enhanced MRA has a sensitivity of 80% to 97%, and a specificity of 73% to 96% (Fig. 92-8).38 The disadvantage of MRA is that it cannot be used in patients with metallic implants such as pacemakers and patients with claustrophobia. Furthermore, in patients with moderately severe renal dysfunction (glomerular filtration rate of <60 mL/min/1.72 m2), concerns for nephrogenic systemic fibrosis secondary to gadolinium limit the use of this technology in this patient population.39
Digital Subtraction Arteriography
Abdominal aortography, with or without demonstration of the arterial tree below the inguinal ligament (“runoff”), is performed after a full workup is completed and may be part of the therapeutic intervention. This procedure is most commonly performed via the femoral artery with the better pulse by means of a retrograde Seldinger technique. When neither femoral artery is available, an upper extremity approach may provide good access. Hemorrhage, local hematoma, pseudoaneurysm, arterial thrombosis, or distal embolism of thrombus or atheromatous debris can occur in up to 0.8% of diagnostic procedures and in up to 6% of therapeutic procedures.40,41 Anteroposterior views of the aortoiliofemoral segments demonstrate the extent of the occlusive process and the pattern of collateral formation. Oblique views of the iliac and femoral arteries are frequently necessary to document posterior wall plaques and stenoses at the origins of the hypogastric and deep femoral arteries. Views of the “runoff” arteries to at least the midcalf level are required to assess the degree of associated infrainguinal occlusive disease. In cases of CLI, visualization of the pedal circulation is also necessary. In patients with borderline kidney function, carbon dioxide may be utilized as a contrast agent.

Figure 92-8. MRA of a patient who had an aortobifemoral bypass. Note the proximal anastomosis (thin arrow) and the distal anastomosis (thick arrows). Also note the retrograde flow into the iliac arteries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



