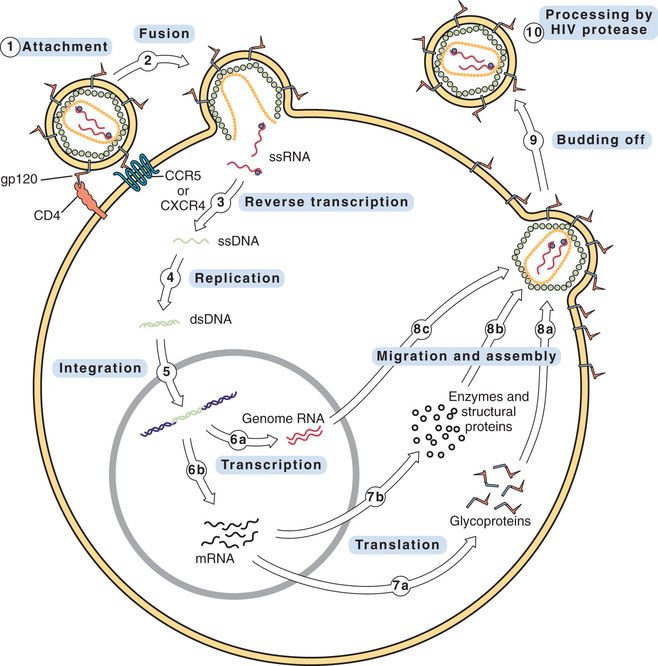
The central core contains two separate but identical single strands of RNA, each with its own molecule of reverse transcriptase attached. The RNA serves as the template for DNA synthesis.
The outer envelope of HIV contains glycoproteins that are needed for attachment to host cells. Each glycoprotein (gp) consists of two subunits, known as gp41 and gp120. The smaller protein (gp41) is embedded in the lipid bilayer of the viral envelope; the larger protein (gp120) is connected firmly to gp41. (The numbers 41 and 120 simply indicate the mass of these glycoproteins in thousands of daltons.)
Replication Cycle of HIV
The replication cycle of HIV is shown in Fig. 79.2. The numbered steps listed here correspond to the numbers in the figure.
• Step 1—The cycle begins with attachment of HIV to the host cell. The primary connection takes place between gp120 on the HIV envelope and a CD4 protein on the host cell membrane. Other host proteins, known as coreceptors, act in concert with CD4 to tighten the bond with HIV. Two of these coreceptors—known as CCR5 and CXCR4—are of particular importance. (One drug—maraviroc—blocks HIV entry by binding CCR5.)
• Step 2—The lipid bilayer envelope of HIV fuses with the lipid bilayer of the host cell membrane. Fusion is followed by release of HIV RNA into the host cell. (One drug—enfuvirtide—works by blocking the fusion process.)
• Step 3—HIV RNA is transcribed into single-stranded DNA by HIV reverse transcriptase.
• Step 4—Reverse transcriptase converts the single strand of HIV DNA into double-stranded HIV DNA.
• Step 5—Double-stranded HIV DNA becomes integrated into the host’s DNA, under the direction of a viral enzyme known (aptly) as integrase. (One drug—raltegravir—inhibits this enzyme.)
• Step 6—HIV DNA undergoes transcription into RNA. Some of the resulting RNA becomes the genome for daughter HIV virions (step 6a). The rest of the RNA is messenger RNA that codes for HIV proteins (step 6b).
• Step 7—Messenger RNA is translated into HIV glycoproteins (step 7a) and HIV enzymes and structural proteins (step 7b).
• Step 8—The components of HIV migrate to the cell surface and assemble into a new virus. Before assembly, HIV glycoproteins become incorporated into the host cell membrane (step 8a). In steps 8b and 8c, the other components of the virion migrate to the cell surface, where they undergo assembly into the new virus.
• Step 9—The newly formed virus buds off from the host cell. As indicated, the outer envelope of the virion is derived from the cell membrane of the host.
• Step 10—In this step, which occurs either during or immediately after budding off, HIV undergoes final maturation under the influence of protease, an enzyme that cleaves certain large polyproteins into their smaller, functional forms. If protease fails to cleave these proteins, HIV will remain immature and noninfectious. HIV protease is the target of several important drugs.
Replication Rate
HIV replicates rapidly during all stages of the infection. During the initial phase of infection, replication is massive. Why? Because (1) the population of CD4 cells is still large, thereby providing a large viral breeding ground; and (2) the host has not yet mounted an immune response against HIV, so replication can proceed unopposed. As a result of massive replication, plasma levels of HIV can exceed 10 million virions/mL. During this stage of high viral load, patients often experience an acute retroviral syndrome (see later).
Over the next few months, as the immune system begins to attack HIV, plasma levels of HIV undergo a sharp decline and then level off. A typical steady-state level is between 1000 and 100,000 virions/mL. Please note, however, that steady-state numbers can be deceptive. The plasma half-life of HIV is only 6 hours; that is, every 6 hours, half of the HIV virions in plasma are lost. Accordingly, to maintain the steady-state levels typically seen during chronic HIV infection, the actual rate of replication is between 1 and 10 billion virions/day. Despite this high rate of ongoing replication, infected persons typically remain asymptomatic for about 10 years, after which symptoms of advanced HIV disease appear.
Mutation and Drug Resistance
HIV mutates rapidly. Why? Because HIV reverse transcriptase is an error-prone enzyme. Therefore, whenever it transcribes HIV RNA into single-stranded DNA and then into double-stranded DNA, there is a high probability of introducing base-pair errors. In fact, according to one estimate, up to 10 incorrect bases may be incorporated into HIV DNA during each round of replication. Because of these errors, HIV can rapidly mutate from a drug-sensitive form into a drug-resistant form. The probability of developing resistance in the individual patient is directly related to the total viral load. Hence the more virions the patient harbors, the greater the likelihood that at least one will become resistant. To minimize the emergence of resistance, patients must be treated with a combination of antiretroviral drugs. This is the same strategy we employ to prevent emergence of resistance when treating tuberculosis (see Chapter 75).
Transmission of HIV
HIV is transmitted sexually and by other means. The virus is present in all body fluids of infected individuals. Transmission can be by intimate contact with semen, vaginal secretions, and blood. The disease can be transmitted by sexual contact, transfusion, sharing intravenous (IV) needles, and accidental needle sticks. In addition, it can be transmitted to the fetus by an infected mother, usually during the perinatal period. Initially, HIV infection was limited largely to homosexual males, injection-drug users, and hemophiliacs. However, the disease can now be found routinely in the population at large. The risk for acquiring HIV sexually can be reduced by male circumcision, limiting sexual partners, and use of condoms—as well as by complete sexual abstinence. In addition, infection can be prevented with drugs, as discussed later under “Preventing HIV Infection with Drugs.”
Clinical Course of HIV Infection
HIV infection follows a triphasic clinical course. During the initial phase, HIV undergoes massive replication, causing blood levels of HIV to rise very high. As a result, between 50% and 90% of patients experience a flu-like acute retroviral syndrome. Signs and symptoms include fever, lymphadenopathy, pharyngitis, rash, myalgia, and headache (Box 79.1). Soon, however, the immune system mounts a counterattack, causing HIV levels to fall. As a result, symptoms of the acute syndrome fade. Very often, the acute retroviral syndrome is perceived as influenza, and so it goes unrecognized for what it really is.
The middle phase of HIV infection is characterized by prolonged clinical latency. Blood levels of HIV remain relatively low, and most patients are asymptomatic. However, as noted previously, HIV continues to replicate despite apparent dormancy. Because of persistent HIV replication, CD4 T cells undergo progressive decline. The average duration of clinical latency is 10 years.
During the late phase of HIV infection, CD4 T cells drop below a critical level (200 cells/mL), rendering the patient highly vulnerable to opportunistic infections and certain neoplasms (e.g., Kaposi sarcoma). The late phase is when AIDS occurs.
Many patients with HIV infection experience neurologic complications. Both the peripheral and central nervous systems may be involved. Peripheral neuropathies affect 20% to 40% of patients and may develop at any time over the course of HIV infection. In contrast, CNS complications usually occur late in the disease. Symptoms of CNS injury include decreased cognition, reduced concentration, memory loss, mental slowness, and motor complaints (e.g., ataxia, tremors). Neuronal injury may be the direct result of HIV infection or may develop secondary to an opportunistic infection in the CNS.
Drug Interactions
Before we begin our discussion of the different classes of antiretroviral drugs, it will be wise to explore a topic of great concern. Drug interactions are common and significant with these drugs. Many are inducers or inhibitors of one or more (and sometimes many) CYP450 isoenzymes. Many are also substrates of one or more of these. As a result, interactions are common. Some drugs have the same adverse effect, and giving them together can intensify an effect so that it becomes dangerous. Moreover, when we consider all the various combinations of these drugs plus drugs taken for other conditions and illnesses the patient may have, the possibility of dangerous drug interactions increases dramatically. Simple lists of common drug interactions are inadequate to address this issue.
Every provider needs access to reliable drug interaction software that is capable of simultaneously checking for interactions among multiple drugs. These are widely available online and as downloads for mobile devices.
Classification of Antiretroviral Drugs
At this time, we have five types of antiretroviral drugs. Three types—reverse transcriptase inhibitors, integrase strand transfer inhibitors (INSTIs), and protease inhibitors (PIs)—inhibit enzymes required for HIV replication. The other two types—fusion inhibitors and chemokine receptor 5 (CCR5) antagonists—block viral entry into cells. As discussed later, the reverse transcriptase inhibitors are subdivided into two groups: nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), which are structural analogs of nucleosides or nucleotides; and (2) nonnucleoside reverse transcriptase inhibitors (NNRTIs). Drugs that belong to these groups are shown in Table 79.1.
TABLE 79.1
Classification of Antiretroviral Drugs
| Generic Name | Trade Name | Abbreviation |
| DRUGS THAT INHIBIT HIV ENZYMES | ||
| Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTIs) | ||
| Single-Drug Products | ||
| Abacavir | Ziagen | ABC |
| Didanosine | Videx | ddI |
| Emtricitabine | Emtriva | FTC |
| Lamivudine | Epivir | 3TC |
| Stavudine | Zerit | d4T |
| Tenofovir | Viread | TDF |
| Zidovudine | Retrovir | ZDV |
| Fixed-Dose Combinations | ||
Abacavir 600 mg Lamivudine 300 mg | Epzicom | ABC/3TC |
Abacavir 300 mg Lamivudine 150 mg Zidovudine 300 mg | Trizivir | ABC/3TC/ZDV |
Zidovudine 300 mg Lamivudine 150 mg | Combivir | ZDV/3TC |
Emtricitabine 200 mg Tenofovir 300 mg | Truvada | FTC/TDF |
Emtricitabine 200 mg Tenofovir 300 mg Efavirenz* 600 mg | Atripla | FTC/TDF/EFV |
Emtricitabine 200 mg Tenofovir 300 mg Rilpivirine† 25 mg | Complera | FTC/TDF/RPV |
Efavirenz* 600 mg Tenofovir 300 mg Emtricitabine 200 mg | Atripla | EFV/TDF/FTC |
Elvitegravir‡ 150 mg Cobicistat§ 150 mg Emtricitabine 200 mg Tenofovir 300 mg | Stribild | N/A |
| Nonnucleoside Reverse Transcriptase Inhibitors (NNRTIs) | ||
| Delavirdine | Rescriptor | DLV |
| Efavirenz | Sustiva | EFV |
| Etravirine | Intelence | ETR |
| Nevirapine | Viramune | NVP |
| Rilpivirine | Edurant | RPV |
| Protease Inhibitors | ||
| Atazanavir | Reyataz | ATV |
| Darunavir | Prezista | DRV |
| Fosamprenavir | Lexiva, Telzir  | FPV |
| Indinavir | Crixivan | IDV |
| Nelfinavir | Viracept | NFV |
| Ritonavir | Norvir | RTV |
| Saquinavir | Invirase | SQV |
| Tipranavir | Aptivus | TPV |
| Lopinavir/ritonavir | Kaletra | LPV/r |
| Integrase Strand Transfer Inhibitor | ||
| Raltegravir | Isentress | RAL |
| Elvitegravir | Vitekta | EVG |
| DRUGS THAT BLOCK HIV ENTRY INTO CELLS | ||
| Fusion Inhibitor | ||
| Enfuvirtide | Fuzeon | T-20 |
| CCR5 Antagonist | ||
| Maraviroc | Selzentry, Celsentri  | MVC |
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors
The NRTIs were the first drugs used against HIV infection and remain mainstays of therapy today. In fact, these drugs constitute the backbone of all treatment regimens. As their name suggests, the NRTIs are chemical relatives of naturally occurring nucleosides or nucleotides, the building blocks of DNA. Antiretroviral effects derive from suppressing synthesis of viral DNA by reverse transcriptase. All are prodrugs; to be effective, all of the NRTIs must first undergo intracellular conversion to their active (triphosphate) forms. At this time, seven NRTIs are available: abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zidovudine. The availability of combination antiretroviral products has simplified treatment. The fixed-dose combinations are shown in Table 79.1. Pharmacokinetic properties of NRTIs are shown in Table 79.2. Significant adverse effects are provided in Table 79.3. Important properties of these drugs, as well as drugs of other categories, are provided in the “Prescribing and Monitoring Considerations” at the end of this chapter.
PATIENT-CENTERED CARE ACROSS THE LIFE SPAN
Nucleoside Reverse Transcriptase Inhibitors
| Life Stage | Considerations or Concerns |
| Children | Pediatric dosing is available for all NRTIs. |
| Pregnant women | Didanosine, emtricitabine, and tenofovir are in FDA Pregnancy Risk Category B. Lamivudine, stavudine, and zidovudine are in FDA Pregnancy Risk Category C. Current labeling for abacavir does not include a risk category. The choice of antiretroviral drug for the pregnant woman must consider not only the risk for harm to the fetus from the drug but also the risk for harm to the fetus from the adverse effects tied to the drug. Although it is categorized in FDA Pregnancy Risk Category C, zidovudine is the drug of choice for preventing mother-to-infant HIV transmission during labor and delivery. |
| Breastfeeding women | Breastfeeding should be avoided by women with HIV because there is a danger of transmitting the virus. |
| Older adults | Older patients taking didanosine have a higher risk for developing pancreatitis than younger patients. Peripheral neuropathy may be increased for older patients taking stavudine. |
TABLE 79.2
Pharmacokinetic Properties of Nucleoside/Nucleotide Reverse Transcriptase Inhibitors
| Abacavir (ABC) | Didanosine (ddI) | Emtricitabine (FTC) | Lamivudine (3TC) | Stavudine (d4T) | Tenofovir (TDF) | Zidovudine (ZDV) | |
| Trade name | Ziagen | Videx, Videx EC | Emtriva | Epivir | Zerit | Viread | Retrovir |
| Bioavailability | 83% | 30%–40% | 93% | 86% | 86% | 39% (with food) | 60% |
| Serum half-life | 1.5 hr | 1.5 hr | 10 hr | 5–7 hr | 1 hr | 17 hr | 1.1 hr |
| Intracellular half-life | 12–26 hr | More than 20 hr | More than 20 hr | 18–22 hr | 7.5 hr | More than 60 hr | 7 hr |
| Elimination | Metabolized by alcohol dehydrogenase, then excreted in the urine | Partial metabolism followed by renal excretion | Renal excretion | Renal excretion (unchanged) | Partial metabolism followed by renal excretion | Renal excretion | Hepatic metabolism followed by renal excretion |
TABLE 79.3
Significant Adverse Effects of Antiretroviral Drugs
| NUCLEOSIDE/NUCLEOTIDE REVERSE TRANSCRIPTASE INHIBITORS | |
| Abacavir [ABC] | Lactic acidosis,* severe hepatomegaly with steatosis,* severe hypersensitivity reactions,* headache, nausea, vomiting, fatigue, malaise, sleep disorders |
| Didanosine [ddI] | Lactic acidosis,* severe hepatomegaly with steatosis,* severe pancreatitis,* hepatotoxicity,† noncirrhotic portal hypertension,† immune reconstitution syndrome,† redistribution of adipose tissue,† peripheral neuropathy,† retinal disorders and/or optic neuritis,† headache, nausea, vomiting, rash |
| Emtricitabine [FTC] | Lactic acidosis,* severe hepatomegaly with steatosis,* hepatitis B exacerbations,* immune reconstitution syndrome,† redistribution of adipose tissue,† headache, nausea, fatigue, malaise, weakness, sleep disorders, depression, rash, dermal hyperpigmentation, rhinitis, cough, abdominal discomfort, diarrhea |
| Lamivudine [3TC] | Lactic acidosis,* severe hepatomegaly with steatosis,* hepatitis B exacerbations,* risk for HIV-1 resistance if used in a patient with untreated HIV-1 infection,* ENT infections, sore throat, diarrhea |
| Stavudine [d4T] | Lactic acidosis,* severe hepatomegaly with steatosis,* severe pancreatitis,* hepatotoxicity,† immune reconstitution syndrome,† redistribution of adipose tissue,† neurologic symptoms (e.g., motor weakness, peripheral neuropathy),† headache, nausea, vomiting, rash, diarrhea |
| Tenofovir [TDF] | Lactic acidosis,* severe hepatomegaly with steatosis,* hepatitis B exacerbations,* immune reconstitution syndrome,† redistribution of adipose tissue,† renal impairment,† decreased BMD,† headache, nausea, weakness, depression, rash, diarrhea |
| Zidovudine [ZDV] | Lactic acidosis,* severe hepatomegaly with steatosis,* severe anemia and neutropenia,* serious myopathy and myositis,* immune reconstitution syndrome,† redistribution of adipose tissue,† headache, nausea, vomiting, anorexia, malaise, fever, cough |
| NONNUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS | |
| Delavirdine [Rescriptor] | Rash ranging from erythema and pruritus (16.7%) to desquamation and ulceration (4.4%), headache, nausea, vomiting, fatigue, weakness, diarrhea |
| Efavirenz [Sustiva] | Rash,† hepatotoxicity,† severe depression with/without suicidal ideation,† nervous system symptoms,† convulsions,† immune reconstitution syndrome,† redistribution of adipose tissue,† hyperlipidemia,† headache, nausea, vomiting, fatigue, dizziness, impaired concentration, sleep disorders |
| Etravirine [Intelence] | Severe rashes, including SJS and TEN,† peripheral neuropathy |
| Nevirapine [Viramune] | Life-threatening skin reactions,* hepatotoxicity,* immune reconstitution syndrome,† redistribution of adipose tissue† |
| Rilpivirine [Edurant] | Rash,† hypersensitivity reactions,† hepatotoxicity,† immune reconstitution syndrome,† redistribution of adipose tissue,† depression,† headache, sleep disorders |
| PROTEASE INHIBITORS | |
| Atazanavir [Reyataz] | Dangerous drug interactions,† severe skin reactions,† PR interval prolongation,† hepatotoxicity,† hyperbilirubinemia,† kidney stones and gallstones,† diabetes (new onset or exacerbation),† immune reconstitution syndrome,† redistribution of adipose tissue,† renewed bleeding in patients with hemophilia,† headache, dizziness, nausea, vomiting, abdominal discomfort, fever, rash, peripheral neurologic symptoms, depression, sleep disorders |
| Darunavir [Prezista] | Dangerous drug interactions,† severe skin reactions, including SJS and TEN, hepatitis, diabetes (new onset or exacerbation),† immune reconstitution syndrome,† redistribution of adipose tissue,† renewed bleeding in patients with hemophilia,† headache, nausea, vomiting, abdominal discomfort, diarrhea, rash |
| Fosamprenavir [Lexiva] | Dangerous drug interactions,† severe skin reactions, including SJS and TEN, transaminase elevations (especially in patients with hepatitis B or C),† diabetes (new onset or exacerbation),† immune reconstitution syndrome,† redistribution of adipose tissue,† hyperlipidemia,† hemolytic anemia,† renewed bleeding in patients with hemophilia,† kidney stones,† headache, nausea, vomiting, diarrhea, rash |
| Indinavir [Crixivan] | Dangerous drug interactions,† hepatitis, including reports of liver failure,† hyperglycemia or diabetes (new onset or exacerbation),† hemolytic anemia,† renewed bleeding in patients with hemophilia,† kidney stones,† hyperbilirubinemia, headache, nausea, vomiting, abdominal pain, back pain |
| Lopinavir (with ritonavir) [Kaletra] | Dangerous drug interactions,† hepatotoxicity,† pancreatitis,† PR interval prolongation,† QT interval prolongation,† hyperglycemia or diabetes (new onset or exacerbation),† immune reconstitution syndrome,† redistribution of adipose tissue,† hyperlipidemia,† renewed bleeding in patients with hemophilia,† headache, nausea, vomiting, abdominal discomfort, indigestion, weakness |
| Nelfinavir [Viracept] | Dangerous drug interactions,† diabetes (new onset or exacerbation),† diarrhea |
| Ritonavir [Norvir] | Dangerous drug interactions,* hepatotoxicity,† pancreatitis,† severe hypersensitivity reactions,† PR interval prolongation,† hyperlipidemia,† diabetes (new onset or exacerbation),† immune reconstitution syndrome,† redistribution of adipose tissue,† renewed bleeding in patients with hemophilia,† nausea, vomiting, abdominal discomfort, diarrhea, fatigue, weakness, paresthesias, rash |
| Saquinavir [Invirase] | Dangerous drug interactions (including danger with ritonavir),† PR interval prolongation,† QT interval prolongation,† diabetes (new onset or exacerbation),† immune reconstitution syndrome,† redistribution of adipose tissue,† renewed bleeding in patients with hemophilia,† exacerbation of comorbid hepatic disease,† hyperlipidemia,† nausea, vomiting, abdominal pain, diarrhea, fatigue |
| Tipranavir [Aptivus] | Hepatotoxicity,* intracranial hemorrhage,* dangerous drug interactions,† renewed bleeding in patients with hemophilia,† serious rash,† hyperglycemia and diabetes (new onset or exacerbation),† immune reconstitution syndrome,† redistribution of adipose tissue,† renewed bleeding in patients with hemophilia,† increased bleeding risk in the absence of hemophilia,† hyperlipidemia,† headache, nausea, vomiting, indigestion, diarrhea |
| INTEGRASE STRAND TRANSFER INHIBITORS | |
| Raltegravir [Isentress] | Immune reconstitution syndrome,† headache, nausea, insomnia, fatigue, weakness, CK elevations |
| Elvitegravir [Viteka] | Immune reconstitution syndrome,† diarrhea |
| HIV FUSION INHIBITORS | |
| Enfuvirtide [Fuzeon] | Injection site reactions (98%),† hypersensitivity,† postinjection bleeding,† immune reconstitution syndrome,† pneumonia, nausea, diarrhea, fatigue |
| CCR5 ANTAGONISTS | |
Maraviroc [Selzentry, Celsentri  ] ] | Hepatotoxicity,* myocardial ischemia or infarction,† orthostatic hypotension (in patients with impaired renal function),† immune reconstitution syndrome,† increased risk for infection,† potential risk for malignancy,† fever, upper respiratory infections, cough, rash, dizziness |