Advise patients to apply the drug with a finger cot or rubber glove to avoid viral transfer to other body sites or other people.
Inform patients with herpes simplex genitalis that acyclovir only decreases symptoms; it does not eliminate the virus and does not produce cure. Advise patients to cleanse the affected area with soap and water 3 to 4 times a day, drying thoroughly after each wash. Advise patients to avoid all sexual contact while lesions are present and to use a condom even when lesions are absent.
Preparations, Dosage, and Administration
Topical Ointment.
Acyclovir [Zovirax] is supplied as a 5% ointment for topical therapy of herpes genitalis and mild mucocutaneous HSV infection in the immunocompromised host. Application is done 6 times a day at 3-hour intervals for 7 days. Patients should use a finger cot or rubber glove to avoid viral transfer to other parts of the body or to other people.
Topical Cream.
Acyclovir [Zovirax] is supplied as a 5% cream for topical therapy of recurrent herpes labialis (cold sores) in patients at least 12 years old. Application is done 5 times a day for 4 days.
Oral.
Oral acyclovir [Zovirax] is available in capsules (200 mg), tablets (400 and 800 mg), and a suspension (200 mg/5 mL). Dosages for patients with normal kidney function are given here. Dosages must be reduced for patients with renal impairment.
• For initial episodes of herpes genitalis, the usual dosage is 400 mg 3 times a day for 7 to 10 days.
• For episodic recurrences of herpes genitalis, the usual dosage is 400 mg 3 times a day for 5 days.
• For long-term suppressive therapy of recurrent genital infections, the usual dosage is 400 mg twice daily for up to 12 months.
• For acute therapy of herpes zoster, the dosage is 800 mg 5 times a day (at 4-hour intervals) for 7 to 10 days.
• For VZV (chickenpox), the dosage is 20 mg/kg (but no more than 800 mg) 4 times a day for 5 days. Treatment should begin at the earliest sign of rash.
Intravenous.
For IV dosing, acyclovir is available in solution (50 mg/mL). Administration is by slow infusion (over 1 hour or more). Parenteral acyclovir must not be given by IV bolus or by intramuscular (IM) or subcutaneous (subQ) injection. To minimize the risk for renal damage, hydrate the patient during the infusion and for 2 hours after. Dosages for patients with normal kidney function are given here. Dosages should be reduced for patients with renal impairment.
• For mucocutaneous HSV infection in the immunocompromised host, the adult dosage is 5 mg/kg infused every 8 hours for 7 days. The dosage for children under 12 years is 10 mg/kg infused every 8 hours for 7 days.
• For VZV infection in the immunocompromised host, the adult dosage is 10 mg/kg infused every 8 hours for 7 days. The dosage for children under 12 years is 20 mg/kg infused every 8 hours for 7 days.
• For severe episodes of herpes genitalis in the immunocompetent host, the adult dosage is 5 to 10 mg/kg infused every 8 hours for 5 to 7 days (or until symptoms resolve). The dosage for children younger than 12 years is 15 to 20 mg/kg/day divided into three doses to be infused every 8 hours for 5 days.
Valacyclovir
Actions and Uses
Valacyclovir [Valtrex], a prodrug form of acyclovir, is approved for management of four conditions: (1) herpes zoster (shingles), (2) herpes simplex genitalis (genital herpes), (3) herpes labialis (cold sores), and (4) varicella (chickenpox). With the exception of herpes labialis, there are limits on use for each condition. For herpes zoster, valacyclovir is indicated only for immunocompetent patients. For varicella, the patients must be immunocompetent children. For herpes simplex genitalis, valacyclovir is indicated for treatment of initial and recurrent episodes for immunocompetent patients; however, for suppressive therapy, this drug is approved for management in immunocompetent and HIV-infected adults with a CD4+ cell count of at least 100 cells/mm3.
Valacyclovir is sometimes used off-label for prophylaxis of HSV, VZV, and CMV infections in patients with cancer. It is also sometimes used for treatment of cancer-related HSV and VZV.
Pharmacokinetics
Oral valacyclovir undergoes rapid absorption followed by rapid and essentially complete conversion to acyclovir. When acyclovir itself is given orally, bioavailability is only 15% to 30%. In contrast, when valacyclovir is given orally, the effective bioavailability of acyclovir is greatly increased—to about 55%. Therefore valacyclovir represents a more efficient way of getting acyclovir into the body. After conversion of valacyclovir to acyclovir, the kinetics are the same as if acyclovir itself had been given.
Adverse Effects
No doubt you noticed the emphasis on using valacyclovir primarily for immunocompetent patients. Why? In some immunocompromised patients, valacyclovir has produced a syndrome known as thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS). This syndrome, which can be fatal, has not occurred in immunocompetent patients. Aside from causing TTP/HUS, valacyclovir is generally well tolerated, producing the same side effects seen with oral acyclovir (e.g., nausea, vomiting, diarrhea, headache, vertigo).
Preparations, Dosage, and Administration
Valacyclovir [Valtrex] is available in 500- and 1000-mg oral capsules. Dosing may be done without regard to meals. In patients with renal impairment, dosages should be reduced.
For patients with herpes zoster, the recommended dosage is 1000 mg 3 times a day for 7 days. Therapy should begin as soon as possible after symptom onset.
For patients with herpes simplex genitalis, the dosage is 1 g twice daily for 10 days (for the initial episode), or 500 mg twice daily for 3 days (for episodic recurrences). For suppressive therapy, the recommended dosage for immunocompetent patients is 500 to 1000 mg once daily and, for patients with HIV infection, 500 mg twice a day.
For patients with herpes labialis, 2 g/dose should be taken 12 hours apart for 1 day. Dosing should begin as soon as possible after onset of symptoms.
For immunocompetent children aged 2 to 18 years with chickenpox, dosage is 20 mg/kg (up to a maximum of 1 g) 3 times a day.
Famciclovir
Famciclovir [Famvir] is a prodrug used to treat acute herpes zoster and genital herpes infection. Benefits are equivalent to those of acyclovir. Adverse effects are minimal.
Pharmacokinetics
Famciclovir undergoes rapid absorption from the gastrointestinal (GI) tract followed by enzymatic conversion to penciclovir, its active form. Food decreases the rate of famciclovir absorption but not the extent. As a result, the amount of penciclovir produced is the same whether famciclovir is taken with or without food. Penciclovir is excreted in the urine, largely unchanged. The plasma half-life of penciclovir is about 2.5 hours. However, the half-life of penciclovir within cells is much longer. In patients with renal impairment, the plasma half-life of penciclovir is prolonged.
Mechanism of Action and Antiviral Spectrum
Penciclovir undergoes intracellular conversion to penciclovir triphosphate, a compound that inhibits viral DNA polymerase, and thereby prevents replication of viral DNA. Under clinical conditions, formation of penciclovir triphosphate requires viral thymidine kinase. As a result, inhibition of DNA synthesis is limited to cells that are infected, leaving most host cells unharmed. In vitro, penciclovir is active against HSV type 1 (HSV-1), HSV-2, and VZV.
Therapeutic Use
Famciclovir is approved for treatment of acute herpes zoster (shingles) and herpes simplex genitalis. In patients with herpes zoster, the drug can decrease the time to full crusting from 7 days down to 5 days. Famciclovir does not decrease the incidence of postherpetic neuralgia but can decrease the duration (from 112 days down to 61 days).
In patients with genital herpes simplex infection, famciclovir is active against the first episode and recurrent episodes. In addition, it can be used for long-term suppression.
Adverse Effects
Famciclovir is very well tolerated. In clinical trials, the only headache and nausea were reported by more than 10% of the subjects. If given in higher than recommended doses, acute renal failure can occur.
Preparations, Dosage, and Administration
Preparations
Famciclovir [Famvir] is supplied in tablets (125, 250, and 500 mg) for oral dosing, with or without food.
Acute Herpes Zoster
The recommended dosage is 500 mg every 8 hours for 7 days. Treatment should start no later than 72 hours after symptom onset. In patients with renal impairment, the dose should be reduced and the interval between doses should be increased to 12 hours or 24 hours, depending on the degree of impairment.
Herpes Simplex Genitalis
For initial episodes, the dosage is 250 mg 3 times a day for 7 to 10 days. For episodic recurrence, there are three dosage regimens available: (1) 125 mg twice a day for 5 days; (2) 500 mg as a single dose on day 1 followed by 250 mg twice a day on day 2, or (3) two 1000-mg doses 12 hours apart. For long-term suppression, the dosage is 250 mg twice daily for a year.
Herpes Labialis
For cold sores that are recurrent, the dosage is a single 1500-mg dose at the first signs of symptoms.
Topical Drugs for Herpes Labialis
We have three topical drugs for recurrent herpes labialis (cold sores). Two of these drugs—penciclovir and docosanol—are discussed next. The third drug—acyclovir—was discussed earlier.
Penciclovir Cream
Penciclovir [Denavir] is a topical drug indicated for recurrent herpes labialis, an infection caused by HSV-1 and HSV-2. The drug suppresses viral replication by inhibiting DNA polymerase, the enzyme that makes DNA. Penciclovir is supplied as a 1% cream to be applied every 2 hours (except when sleeping) for 4 days. In clinical trials, benefits were modest: the average time to healing and duration of pain were decreased by just half a day, from 5 days down to 4.5 days. The only common adverse effect is mild local erythema.
Docosanol Cream
Docosanol [Abreva] is a topical preparation indicated for recurrent herpes labialis. The drug is available over the counter as a 10% cream. Application is done 5 times a day, beginning at the first sign of recurrence. Benefits are modest. In one trial, treatment reduced the time to healing from 4.8 days down to 4.1 days—about the same response seen with penciclovir. Docosanol cream appears devoid of adverse effects.
Docosanol has a broad antiviral spectrum and a unique mechanism of action. Unlike penciclovir, which inhibits viral DNA synthesis (and thereby suppresses replication), docosanol blocks viral entry into host cells. The drug does not kill viruses and does not prevent them from binding to cells. As a result, viable virions can remain attached to the cell surface for a long time. Because docosanol does not affect processes of replication, it is unlikely to promote resistance.
Topical Drugs for Ocular Herpes Infections
Trifluridine Ophthalmic Solution
Trifluridine [Viroptic] is indicated only for topical treatment of ocular infections caused by HSV-1 and HSV-2. The drug is given to treat acute keratoconjunctivitis and recurrent epithelial keratitis. Antiviral actions result from inhibiting DNA synthesis. The most common side effects are localized burning and stinging. Edema of the eyelid occurs in about 3% of patients. Systemic absorption is minimal after topical administration, so the drug is devoid of systemic toxicity. Trifluridine is supplied as a 1% ophthalmic solution. Treatment consists of placing 1 drop on the cornea every 2 hours while the patient is awake, for a maximum of 9 drops/day. After reepithelialization of the cornea has occurred, the dosage is reduced to 1 drop every 4 hours and continues for an additional 7 days.
Ganciclovir Gel
Ganciclovir 0.15% ophthalmic gel [Zirgan] is indicated for acute herpetic keratitis (inflammation and ulceration of the cornea caused by infection with a herpes simplex virus). As discussed later (see “Ganciclovir”), benefits derive from suppressing viral replication. Principal adverse effects are blurred vision, eye irritation, and red eyes. Systemic effects are absent. The recommended dosage is 1 drop in the affected eye 5 times a day until symptoms abate, followed by 1 drop 3 times a day for 7 days. Instruct patients to apply drops directly to the affected eye and to avoid contact lenses until lesions heal.
Drugs for Cytomegalovirus Infection
Cytomegalovirus is a member of the herpesvirus group, which includes HSV-1 and HSV-2, VZV (the cause of chickenpox), and Epstein-Barr virus (the cause of infectious mononucleosis). Transmission of CMV occurs person to person—through direct contact with saliva, urine, blood, tears, breast milk, semen, and other body fluids. Infection can also be acquired by way of blood transfusion or organ transplantation. Infection with CMV is very common: between 50% and 85% of Americans 40 years and older harbor the virus. After the initial infection, which has minimal symptoms in healthy people, the virus remains dormant within cells for life, without causing detectable injury or clinical illness. Hence, for most healthy people, CMV infection is of little concern. By contrast, people who are immunocompromised—owing to HIV infection, cancer chemotherapy, or use of immunosuppressive drugs—are at high risk for serious morbidity and even death, both from initial CMV infection and from reactivation of dormant CMV. Common sites for infection are the lungs, eyes, and GI tract. Among people with AIDS, CMV retinitis is the principal reason for loss of vision (see Chapter 79). The four drugs used against CMV are discussed next.
Ganciclovir
Ganciclovir [Cytovene, Vitrasert, Zirgan] is a synthetic antiviral agent with activity against herpesviruses, including CMV. Because the drug can cause serious adverse effects, especially granulocytopenia and thrombocytopenia, it should be used only for prevention and treatment of CMV infection in the immunocompromised host.
Mechanism of Action
Ganciclovir is converted to its active form, ganciclovir triphosphate, inside infected cells. As ganciclovir triphosphate, it suppresses replication of viral DNA by (1) inhibiting viral DNA polymerase and (2) undergoing incorporation into the growing DNA chain, which causes premature chain termination.
Pharmacokinetics
Bioavailability of oral ganciclovir is low: only 5% under fasting conditions and 9% when taken with food. When in the blood, the drug is widely distributed to body fluids and tissues. Ganciclovir is excreted unchanged in the urine. In patients with normal renal function, the half-life is about 3 hours. In patients with renal impairment, the half-life is prolonged. Accordingly, dosages should be reduced in patients with kidney disease.
Therapeutic Use
Ganciclovir is approved only to prevent and treat CMV infection in immunocompromised patients, including transplant recipients, those with HIV infection, and those receiving immunosuppressive drugs.
In patients with AIDS, CMV retinitis has an incidence of 15% to 40%. Although most AIDS patients respond initially, the relapse rate is high. Accordingly, for most patients, maintenance therapy should continue indefinitely. The risk for relapse is higher with oral ganciclovir than with IV ganciclovir. Because viral resistance can develop during treatment, this possibility should be considered if the patient responds poorly.
Adverse Effects
Granulocytopenia and Thrombocytopenia
The adverse effect of greatest concern is bone marrow suppression, which can result in granulocytopenia and thrombocytopenia. These effects, which are usually reversible, are more likely with IV therapy than with oral therapy. These hematologic responses can be exacerbated by concurrent therapy with zidovudine. Conversely, granulocytopenia can be reduced with granulocyte colony-stimulating factors. Because of the risk for adverse hematologic effects, blood cell counts must be monitored. Treatment should be interrupted if the absolute neutrophil count falls below 500/mm3 or if the platelet count falls below 25,000/mm3. Cell counts usually begin to recover within 3 to 5 days. Ganciclovir should be used with caution in patients with preexisting cytopenias, in those with a history of cytopenic reactions to other drugs, and in those taking other bone marrow suppressants (e.g., zidovudine).
Reproductive Toxicity
Ganciclovir is teratogenic and embryotoxic in laboratory animals and probably in humans. Women should be advised to avoid pregnancy during therapy and for 90 days after ending treatment. At doses equivalent to those used therapeutically, ganciclovir inhibits spermatogenesis in mice; sterility is reversible with low doses and irreversible with high doses. Female infertility may also occur. Patients should be forewarned of these effects.
Other Adverse Effects
Incidental effects include nausea, fever, rash, anemia, liver dysfunction, and confusion and other central nervous system (CNS) symptoms.
Preparations, Dosage, and Administration
Intravenous
Ganciclovir [Cytovene] is available as a powder (500 mg) to be reconstituted for IV infusion. Solutions are alkaline and must be infused into a freely flowing vein to avoid local injury. For treatment of CMV retinitis, the initial dosage for adults with normal renal function is 5 mg/kg (infused over 1 hour) every 12 hours for 14 to 21 days. Two maintenance dosages can be used: (1) 5 mg/kg infused over 1 hour once every day of the week or (2) 6 mg/kg infused over 1 hour once a day, 5 days a week. Dosages must be reduced for patients with renal impairment. Because many patients with AIDS must continue maintenance therapy for life, they need a permanent IV access and equipment for home infusion. Adequate hydration must be maintained in all patients to ensure renal excretion of ganciclovir.
Oral
Ganciclovir is supplied in 250- and 500-mg tablets for maintenance therapy in patients with CMV retinitis. The usual dosage is 1000 mg 3 times daily with food.
Ocular Implant
The ganciclovir ocular implant [Vitrasert] is indicated for CMV retinitis in patients with AIDS. Surgical implantation, which takes about 1 hour, is performed under local anesthesia on an outpatient basis. Vision is usually blurred for 2 to 4 weeks after the procedure. The implant must be replaced every 5 to 8 months. Clinical trials indicate that CMV retinitis progresses more slowly in patients who receive intraocular ganciclovir compared with those on IV ganciclovir.
Ocular Gel
As discussed earlier (under “Topical Drugs for Ocular Herpes Infections”), ganciclovir is available in a 0.15% gel, marketed as Zirgan, for treating herpetic keratitis.
Valganciclovir
Basic and Clinical Pharmacology
Valganciclovir [Valcyte] is a prodrug version of ganciclovir [Cytovene] with greater oral bioavailability (60% vs. 9%). After absorption from the GI tract, valganciclovir is rapidly metabolized to ganciclovir, its active form—and eventually undergoes excretion as unchanged ganciclovir in the urine. Indications are CMV retinitis and prevention of CMV disease in high-risk organ transplant recipients. In patients with active CMV retinitis, oral valganciclovir is just as effective as intravenous ganciclovir—and much more convenient.
Adverse effects are the same as with ganciclovir. The principal concern is blood dyscrasias—granulocytopenia, anemia, and thrombocytopenia—secondary to bone marrow suppression. In addition, any of the following adverse effects typically occur in 20% to 40% of patients: diarrhea, nausea, vomiting, fever, and headache. Valganciclovir is presumed to pose the same risks for mutagenesis, aspermatogenesis, and carcinogenesis as ganciclovir.
Preparations, Dosage, and Administration
Valganciclovir [Valcyte] is available in (1) 450-mg tablets and (2) a powder that makes a 50-mg/mL oral solution when reconstituted with 91 mL of purified water. All doses should be taken with food to enhance bioavailability.
For treatment of CMV retinitis, the adult dosage is 900 mg twice daily for 21 days, followed by 900 mg once daily for maintenance. Dosage must be reduced for patients with renal impairment.
For prevention of CMV disease in transplant recipients, the adult dosage is 900 mg once daily, starting within 10 days of transplantation and continuing until 100 days posttransplantation (or 200 days after transplantation in kidney recipients).
Because valganciclovir has the potential for mutagenesis and carcinogenesis, the powder and tablets should be handled carefully. Tablets should be ingested intact, without crushing or chewing. Direct contact with the powder or broken tablets should be avoided. If contact does occur, the area should be washed with soap and water. When handling or disposing of the drug, healthcare workers should follow the same guidelines established for cytotoxic anticancer drugs.
Cidofovir
Cidofovir [Vistide] is an IV drug with just one indication: CMV retinitis in patients with AIDS who have failed on ganciclovir or foscarnet. Alternative drugs for this infection are foscarnet, which is given intravenously, and ganciclovir, which may be administered intravenously, orally, or by ocular insert. Compared with IV foscarnet or IV ganciclovir, cidofovir has the distinct advantage of needing fewer infusions: Whereas foscarnet and ganciclovir must be infused daily, cidofovir is infused just once a week or every other week. The major adverse effect of the drug is kidney damage.
Mechanism of Action
When inside cells, cidofovir is converted to cidofovir diphosphate, its active form. As the diphosphate, cidofovir causes selective inhibition of viral DNA polymerase and thereby inhibits viral DNA synthesis. Intracellular concentrations of cidofovir diphosphate are too low to inhibit human DNA polymerases; thus host cells are spared.
Antiviral Spectrum and Therapeutic Use
Cidofovir is active against herpesviruses, including CMV, HSV-1, HSV-2, and VZV. However, the drug is approved only for CMV retinitis in patients with AIDS. Whether cidofovir is active against CMV infections in other patients or at other sites (e.g., GI tract, lungs) is unknown. In clinical trials in patients with AIDS and established CMV retinitis, cidofovir significantly delayed progression of retinitis.
Pharmacokinetics
Cidofovir is administered by IV infusion and undergoes excretion by the kidneys. Probenecid competes with cidofovir for renal tubular secretion and thereby delays elimination. Cidofovir has a prolonged intracellular half-life (17–65 hours), and hence a long interval (2 weeks) can separate doses. In contrast, IV foscarnet and ganciclovir must be infused daily.
Adverse Effects
Nephrotoxicity
The principal adverse effect is dose-dependent nephrotoxicity, manifesting as decreased renal function and symptoms of a Fanconi-like syndrome (proteinuria, glucosuria, bicarbonate wasting). To reduce the risk for renal injury, all patients must receive probenecid and IV hydration therapy with each infusion. Also, serum creatinine and urine protein should be checked within 48 hours before each dose, and, if these values indicate kidney damage, cidofovir should be withheld or the dosage reduced. Cidofovir is contraindicated for patients taking other drugs that can injure the kidney and for patients with proteinuria (2+ or greater) or baseline serum creatinine greater than 1.5 mg/dL.
Other Adverse Effects
Neutropenia develops in about 20% of patients, so neutrophil counts should be monitored. Ocular disorders—iritis, uveitis, or ocular hypotony (low intraocular pressure)—can also occur. In animal studies, cidofovir was carcinogenic and teratogenic and caused hypospermia. Adverse effects are more likely in patients taking antiretroviral drugs (i.e., drugs for HIV).
Preparations, Dosage, and Administration
Cidofovir [Vistide] is supplied in solution (75 mg/mL) in 5-mL ampules. To reduce the risk for renal injury, cidofovir infusions must be accompanied by IV hydration therapy and oral (PO) probenecid.
Each cidofovir dose—for induction or maintenance—consists of 5 mg/kg by IV infusion over 1 hour. For induction, two doses are given 1 week apart. For maintenance, one dose is given every 2 weeks. The size of each dose must be reduced for patients with renal impairment. If impairment is severe, cidofovir should be withheld.
Oral probenecid must accompany each infusion. The dosage is 2 g given 3 hours before the infusion, 1 g given 1 hour after the infusion, and another 1 g given 8 hours after that. Ingesting food before each dose can decrease probenecid-induced nausea and vomiting. An antiemetic may also be used.
Hydration is accomplished by infusing 1 L of 0.9% saline solution over 1 to 2 hours immediately before infusing cidofovir. For patients who can tolerate it, 1 L more can be infused over 1 to 3 hours, beginning when the cidofovir infusion begins or as soon as it is over.
Foscarnet
Foscarnet is an IV drug active against all known herpesviruses, including CMV, HSV-1, HSV-2, and VZV. Compared with ganciclovir, foscarnet is more difficult to administer, less well tolerated, and much more expensive. The major adverse effect is renal injury.
Mechanism of Action
Foscarnet, an analog of pyrophosphate, inhibits viral DNA polymerases and reverse transcriptases and thereby inhibits synthesis of viral nucleic acids. At the concentrations achieved clinically, the drug does not inhibit host DNA replication. Unlike many other antiviral drugs, which must undergo conversion to an active form, foscarnet is active as administered.
Therapeutic Use
Foscarnet has two approved indications: (1) CMV retinitis in patients with AIDS and (2) acyclovir-resistant mucocutaneous HSV and VZV infection in the immunocompromised host. CMV retinitis resistant to ganciclovir may respond to foscarnet.
Pharmacokinetics
Foscarnet has low oral bioavailability and must be administered intravenously. The drug is poorly soluble in water and does not penetrate cells easily. As a result, it must be given in large doses with large volumes of fluid. Between 10% and 28% of each dose is deposited in bone; the remainder is excreted unchanged in the urine. Because foscarnet is eliminated by the kidneys, dosages must be reduced in patients with renal impairment. The plasma half-life is 3 to 5 hours.
Adverse Effects and Interactions
In general, foscarnet is less well tolerated than ganciclovir. However, unlike ganciclovir, foscarnet does not cause granulocytopenia or thrombocytopenia.
Nephrotoxicity
Renal injury, as evidenced by a rise in serum creatinine, is the most common dose-limiting toxicity. Most patients develop some degree of renal impairment. Renal injury occurs most often during the second week of therapy. The risk for nephrotoxicity is increased by concurrent use of other nephrotoxic drugs, including amphotericin B, aminoglycosides (e.g., gentamicin), and pentamidine. Prehydration with IV saline may reduce the risk for renal injury. Renal function (creatinine clearance) should be monitored closely, and the dosage should be reduced if renal impairment develops.
Electrolyte and Mineral Imbalances
Foscarnet frequently causes hypocalcemia, hypokalemia, hypomagnesemia, and hypophosphatemia or hyperphosphatemia. Ionized serum calcium may be reduced despite normal levels of total serum calcium. Patients should be informed about symptoms of low ionized calcium (e.g., paresthesias, numbness in the extremities, perioral tingling) and instructed to report these. Severe hypocalcemia can result in dysrhythmias, tetany, and seizures. Serum levels of calcium, magnesium, potassium, and phosphorus should be measured frequently. Special caution is required in patients with preexisting electrolyte, cardiac, or neurologic abnormalities. The risk for hypocalcemia is increased by concurrent use of pentamidine.
Other Adverse Effects
Common reactions (occurring in 25%–50% of patients) include fever, nausea, anemia, diarrhea, vomiting, and headache. In addition, foscarnet can cause fatigue, tremor, irritability, genital ulceration, abnormal liver function tests, neutropenia, and seizures.
Preparations, Dosage, and Administration
Foscarnet is supplied in solution (24 mg/mL) for IV infusion. An infusion pump is essential to reduce the risk for dosing errors. Infusions may be administered through a central venous line or a peripheral vein. When a central line is used, a concentrated (24 mg/mL) solution may be given. When a peripheral vein is used, the solution should be diluted to 12 mg/mL. For patients with normal kidney function, the initial dosage is 60 mg/kg (for CMV infection) or 40 mg/kg (for HSV infection) infused over 1 hour (or longer) every 8 hours for 2 to 3 weeks. The maintenance dosage (for CMV or HSV infection) is 90 to 120 mg/kg infused over 2 hours once daily. All dosages must be reduced for patients with renal impairment.
Drugs for Hepatitis
Viral hepatitis is the most common liver disorder, affecting millions of Americans. The disease can be caused by six different hepatitis viruses, labeled A, B, C, D, E, and G. All six can cause acute hepatitis, but only B, C, and D also cause chronic hepatitis. Acute hepatitis lasts for 6 months or less and is characterized by liver inflammation, jaundice, and elevation of serum alanine aminotransferase (ALT) activity. In most cases, acute hepatitis resolves spontaneously, so intervention is generally unnecessary. In contrast, chronic hepatitis can lead to cirrhosis, hepatocellular carcinoma, and life-threatening liver failure, and hence treatment should be considered.
Most cases (90%) of chronic hepatitis are caused by either hepatitis B virus (HBV) or hepatitis C virus (HCV). Accordingly, our discussion focuses on hepatitis B and hepatitis C. About 1.5% of Americans are infected with HBV or HCV, which is 5 times more than the number infected with HIV. Vaccines for hepatitis A and B are discussed in Chapter 53. Drugs for hepatitis B and C are discussed here.
Hepatitis C
The Centers for Disease Control and Prevention (CDC) estimate that about 3.9 million Americans have chronic hepatitis C. Transmission occurs primarily through exchange of blood, with injection drug use being the most common means. Transmission may also occur as the result of sex with an HCV-infected partner, although this occurs far less frequently. Pregnant women who are infected can transfer the virus to their offspring. Among people who acquire HCV, 75% to 85% develop active infection. However, most people with chronic hepatitis C have no symptoms, although they can transmit HCV to others. Chronic HCV infection undergoes slow progression and, in some people, eventually causes liver failure, cancer, and death. Chronic hepatitis C is the leading reason for liver transplantations and kills about 15,000 Americans each year—more than are killed by HIV.
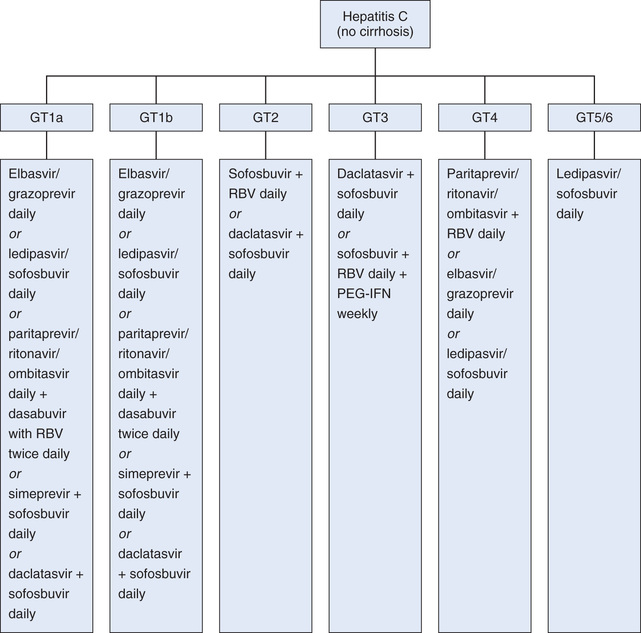
It is important to note that not all hepatitis C viruses are the same. There are 6 genotypes of HCV, and more than 50 subtypes. In the United States 75% of HCV infections are caused by HCV genotype 1, which, unfortunately, is less responsive to treatment than other HCV genotypes.
The options for hepatitis C management increased dramatically between 2011 and 2016 as new categories of antiviral drugs were developed and added to the arsenal of agents targeting HCV infection. New guidelines were developed and then updated and then updated yet again. In 2015 the European Association for the Study of Liver (EASL) released their groundbreaking recommendations for treatment of HCV infection (available online at http://www.easl.eu/medias/cpg/HEPC-2015/Full-report.pdf). The first sentence following the introduction was astounding: “The primary goal of HCV therapy is to cure the infection.” For the patients, their families, and the health care providers who had accepted the long-held belief that there is no cure, hope had truly arrived.
At the time of this writing, joint guidelines by the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) have just been released (see http://www.hcvguidelines.org). These guidelines complement those of the EASL. Like the EASL guidelines, they focus on genotype-specific treatment that considers liver status (i.e., presence of cirrhosis) and treatment history (i.e., treatment-naïve and previous treatment failure) to optimize therapy. (See Fig. 78.1.)

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Black Box Warning: Ganciclovir and Its Prodrug Valganciclovir
Black Box Warning: Ganciclovir and Its Prodrug Valganciclovir Black Box Warning: Cidofovir [Vistide]
Black Box Warning: Cidofovir [Vistide] Black Box Warning: Foscarnet [Foscavir]
Black Box Warning: Foscarnet [Foscavir]