Stage Two: Coagulation
Coagulation is defined as production of fibrin, a thread-like protein that reinforces the platelet plug. Fibrin is produced by two convergent pathways (Fig. 44.2), referred to as the contact activation pathway (also known as the intrinsic pathway) and the tissue factor pathway (also known as the extrinsic pathway). The two pathways converge at factor Xa, after which they employ the same final series of reactions. In both pathways, each reaction in the sequence amplifies the reaction that follows. Hence, after this sequence is initiated, it becomes self-sustaining and self-reinforcing.
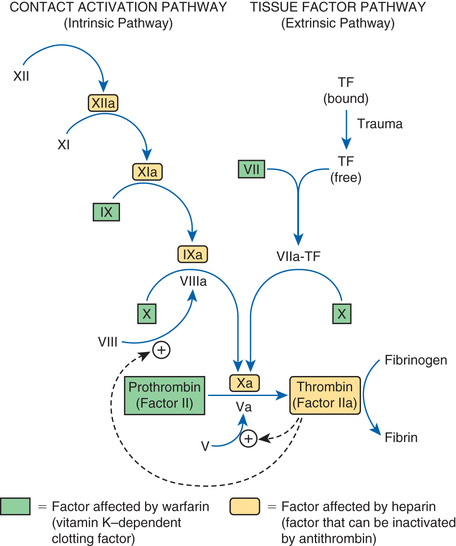
The tissue factor pathway is turned on by trauma to the vascular wall, which triggers release of tissue factor,1 also known as tissue thromboplastin. Tissue factor then combines with and thereby activates factor VII, which in turn activates factor X, which then catalyzes the conversion of prothrombin (factor II) into thrombin (factor IIa). Thrombin then does three things. First, it catalyzes the conversion of fibrinogen into fibrin. Second, it catalyzes the conversion of factor V into its active form (Va), a compound that greatly increases the activity of factor Xa, even though it has no direct catalytic activity of its own. Third, thrombin catalyzes the conversion of factor VIII into its active form (VIIIa), a compound that greatly increases the activity of factor IXa in the contact activation pathway.
The contact activation pathway is turned on when blood makes contact with collagen that has been exposed as a result of trauma to a blood vessel wall. Collagen contact stimulates conversion of factor XII into its active form, XIIa (see Fig. 44.2). Factor XIIa then activates factor XI, which activates factor IX, which activates factor X. After this, the contact activation pathway is the same as the tissue factor pathway. As noted, factor VIIIa, which is produced under the influence of thrombin, greatly increases the activity of factor IXa, even though it has no direct catalytic activity of its own.
Important to our understanding of anticoagulant drugs is the fact that four coagulation factors—factors VII, IX, X, and II (prothrombin)—require vitamin K for their synthesis. These factors appear in green boxes in Fig. 44.2. The significance of the vitamin K–dependent factors will become apparent when we discuss warfarin, an oral anticoagulant.
Keeping Hemostasis Under Control
To protect against widespread coagulation, the body must inactivate any clotting factors that stray from the site of vessel injury. Inactivation is accomplished with antithrombin, a protein that forms a complex with clotting factors and thereby inhibits their activity. The clotting factors that can be neutralized by antithrombin appear in yellow in Fig. 44.2. As we shall see, antithrombin is intimately involved in the action of heparin, an injectable anticoagulant drug.
Physiologic Removal of Clots
As healing of an injured vessel proceeds, removal of the clot is eventually necessary. The body accomplishes this with plasmin, an enzyme that degrades the fibrin meshwork of the clot. Plasmin is produced through the activation of its precursor, plasminogen. The fibrinolytic drugs (e.g., alteplase) act by promoting conversion of plasminogen into plasmin.
Thrombosis
A thrombus is a blood clot formed within a blood vessel or within the heart. Thrombosis (thrombus formation) reflects pathologic functioning of hemostatic mechanisms.
Arterial Thrombosis
Formation of an arterial thrombus begins with adhesion of platelets to the arterial wall. (Adhesion is stimulated by damage to the wall or rupture of an atherosclerotic plaque.) After adhesion, platelets release ADP and thromboxane A2 (TXA2), and thereby attract additional platelets to the evolving thrombus. With continued platelet aggregation, occlusion of the artery takes place. As blood flow comes to a stop, the coagulation cascade is initiated, causing the original plug to undergo reinforcement with fibrin. The consequence of an arterial thrombus is localized tissue injury owing to lack of perfusion.
Venous Thrombosis
Venous thrombi develop at sites where blood flow is slow. Stagnation of blood initiates the coagulation cascade, resulting in the production of fibrin, which enmeshes red blood cells and platelets to form the thrombus. The typical venous thrombus has a long tail that can break off to produce an embolus. Such emboli travel within the vascular system and become lodged at faraway sites, frequently the pulmonary arteries. Hence, unlike an arterial thrombus, whose harmful effects are localized, injury from a venous thrombus occurs secondary to embolization at a site distant from the original thrombus.
Overview of Drugs for Thromboembolic Disorders
The drugs considered fall into three major groups: (1) anticoagulants, (2) antiplatelet drugs, and (3) thrombolytic drugs, also known as fibrinolytic drugs. Anticoagulants (e.g., heparin, warfarin, dabigatran) disrupt the coagulation cascade and thereby suppress production of fibrin. Antiplatelet drugs (e.g., aspirin, clopidogrel) inhibit platelet aggregation. Thrombolytic drugs (e.g., alteplase) promote lysis of fibrin, causing dissolution of thrombi. Because these drugs are used only in a hospital setting, discussion of thrombolytics occurs in Chapter 89. Drugs that belong to these groups are shown in Table 44.1.
TABLE 44.1
Overview of Drugs for Thromboembolic Disorders
| Generic Name | Trade Name | Route | Action | Therapeutic Use |
| ANTICOAGULANTS | Anticoagulants decrease formation of fibrin | Used primarily to prevent thrombosis in veins and the atria of the heart | ||
| Vitamin K Antagonist | ||||
| Warfarin | Coumadin | PO | ||
| Heparin and Its Derivatives: Drugs That Activate Antithrombin | ||||
| Heparin (unfractionated) | SubQ, IV | |||
| LMW heparins | ||||
| Dalteparin | Fragmin | SubQ | ||
| Enoxaparin | Lovenox | SubQ | ||
| Fondaparinux | Arixtra | SubQ | ||
| Direct Thrombin Inhibitors | ||||
| Hirudin Analogs | ||||
| Desirudin | Iprivask | SubQ | ||
| Other Direct Thrombin Inhibitors | ||||
| Dabigatran | Pradaxa, Pradax  | PO | ||
| Direct Factor Xa Inhibitors | ||||
| Rivaroxaban | Xarelto | PO | ||
| Apixaban | Eliquis | PO | ||
| Edoxaban | Savaysa | PO | ||
| ANTIPLATELET DRUGS | Antiplatelet drugs suppress platelet aggregation | Used primarily to prevent thrombosis in arteries | ||
| Cyclooxygenase Inhibitor | ||||
| Aspirin | PO | |||
| P2Y12 Adenosine Diphosphate Receptor Antagonists | ||||
| Clopidogrel | Plavix | PO | ||
| Prasugrel | Effient | PO | ||
| Ticagrelor | Brilinta | PO | ||
| Generic only | PO | |||
| Protease-Activated Receptor-1 (PAR-1) Antagonists | ||||
| Vorapaxar | Zontivity | PO | ||
| Other Antiplatelet Drugs | ||||
| Dipyridamole | Persantine | PO | ||
| Cilostazol | Pletal | PO | ||
Although the anticoagulants and the antiplatelet drugs both suppress thrombosis, they do so by different mechanisms. As a result, they differ in their effects and applications. The antiplatelet drugs are most effective at preventing arterial thrombosis, whereas anticoagulants are most effective against venous thrombosis.
Anticoagulants
By definition, anticoagulants are drugs that reduce formation of fibrin. Two basic mechanisms are involved. One anticoagulant—warfarin—inhibits the synthesis of clotting factors, including factor X and thrombin. All other anticoagulants inhibit the activity of clotting factors: either factor Xa, thrombin, or both.
Anticoagulants are in three pharmacologic classes—vitamin K antagonists, direct factor Xa inhibitors, and direct thrombin inhibitors (see Table 44.1).
Heparin and Its Derivatives: Drugs That Activate Antithrombin
All drugs in this group share the same mechanism of action. Specifically, they greatly enhance the activity of antithrombin, a protein that inactivates two major clotting factors: thrombin and factor Xa. In the absence of thrombin and factor Xa, production of fibrin is reduced, and hence clotting is suppressed.
Our discussion focuses on three preparations: unfractionated heparin, the low-molecular-weight (LMW) heparins, and fondaparinux. Although all three activate antithrombin, they do not have equal effects on thrombin and factor Xa. Specifically, heparin reduces the activity of thrombin and factor Xa more or less equally; the LMW heparins reduce the activity of factor Xa more than they reduce the activity of thrombin; and fondaparinux causes selective inhibition of factor Xa, having no effect on thrombin. Properties of the three preparations are shown in Table 44.2.
TABLE 44.2
Comparison of Drugs That Activate Antithrombin
| Property | Unfractionated Heparin | Low-Molecular-Weight Heparins | Fondaparinux |
| Molecular weight range | 3000–30,000 | 1000–9000 | 1728 |
| Mean molecular weight | 12,000–15,000 | 4000–5000 | 1728 |
| Mechanism of action | Activation of antithrombin, resulting in the inactivation of factor Xa and thrombin | Activation of antithrombin, resulting in preferential inactivation of factor Xa, plus some inactivation of thrombin | Activation of antithrombin, resulting in selective inactivation of factor Xa |
| Routes | IV, subQ | SubQ only | SubQ only |
| Nonspecific binding | Widespread | Minimal | Minimal |
| Laboratory monitoring | aPTT monitoring is essential | No aPTT monitoring required | No aPTT monitoring required |
| Dosage | Dosage must be adjusted on the basis of aPTT | Dosage is fixed | Dosage is fixed |
| Setting for use | Hospital | Hospital or home | Hospital or home |
| Cost | $3/day for heparin itself, but hospitalization and aPTT monitoring greatly increase the real cost | $35/day for LMW heparin (enoxaparin [Lovenox]), but home use and absence of aPTT monitoring greatly reduce the real cost | $59/day for fondaparinux, but home use and absence of aPTT monitoring greatly reduce the real cost |
Heparin (Unfractionated)
Heparin is a rapid-acting anticoagulant administered only by injection. Heparin differs from warfarin (an oral anticoagulant) in several respects, including mechanism, time course, indications, and management of overdose.
Chemistry
Heparin is not a single molecule, but rather a mixture of long polysaccharide chains, with molecular weights that range from 3000 to 30,000. The active region is a unique pentasaccharide (five-sugar) sequence found randomly along the chain. An important feature of heparin’s structure is the presence of many negatively charged groups. Because of these negative charges, heparin is highly polar and hence cannot readily cross membranes.
Mechanism of Anticoagulant Action
Heparin suppresses coagulation by helping antithrombin inactivate clotting factors, primarily thrombin and factor Xa. As shown in Fig. 44.3, binding of heparin to antithrombin produces a conformational change in antithrombin that greatly enhances its ability to inactivate both thrombin and factor Xa. However, the process of inactivating these two clotting factors is distinct. To inactivate thrombin, heparin must simultaneously bind with both thrombin and antithrombin, thereby forming a ternary complex. In contrast, to inactivate factor Xa, heparin binds only with antithrombin; heparin itself does not bind with factor Xa.
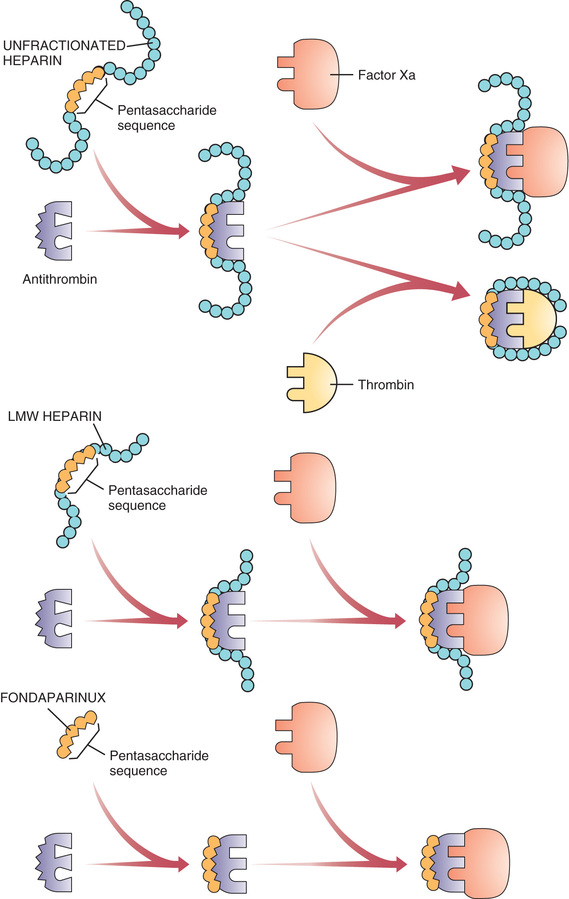
By activating antithrombin, and thereby promoting the inactivation of thrombin and factor Xa, heparin ultimately suppresses formation of fibrin. Because fibrin forms the framework of thrombi in veins, heparin is especially useful for prophylaxis of venous thrombosis. Because thrombin and factor Xa are inhibited as soon as they bind with the heparin-antithrombin complex, the anticoagulant effects of heparin develop quickly (within minutes of intravenous [IV] administration). This contrasts with warfarin, whose full effects are not seen for days.
Pharmacokinetics
Absorption and Distribution.
Because of its polarity and large size, heparin is unable to cross membranes, including those of the gastrointestinal (GI) tract. Consequently, heparin cannot be absorbed if given orally and therefore must be given by injection (IV or subcutaneous [subQ]). Because it cannot cross membranes, heparin does not traverse the placenta and does not enter breast milk.
Protein and Tissue Binding.
Heparin binds nonspecifically to plasma proteins, mononuclear cells, and endothelial cells. As a result, plasma levels of free heparin can be highly variable. Because of this variability, intensive monitoring is required (see later).
Metabolism and Excretion.
Heparin undergoes hepatic metabolism followed by renal excretion. Under normal conditions, the half-life is short (about 1.5 hours). However, in patients with hepatic or renal impairment, the half-life is increased.
Time Course.
Therapy is sometimes initiated with a bolus IV injection, and effects begin immediately. Duration of action is brief (hours) and varies with dosage. Effects are prolonged in patients with hepatic or renal impairment.
Therapeutic Uses
Heparin is a preferred anticoagulant for use during pregnancy (because it doesn’t cross the placenta) and in situations that require rapid onset of anticoagulant effects, including pulmonary embolism (PE) and massive deep vein thrombosis (DVT). In addition, heparin is used for patients undergoing open heart surgery and renal dialysis; during these procedures, heparin serves to prevent coagulation in devices of extracorporeal circulation (heart-lung machines, dialyzers). Low-dose therapy is used to prevent postoperative venous thrombosis. Heparin may also be useful for treating disseminated intravascular coagulation, a complex disorder in which fibrin clots form throughout the vascular system and in which bleeding tendencies may be present; bleeding can occur because massive fibrin production consumes available supplies of clotting factors. Heparin is also used as an adjunct to thrombolytic therapy of acute myocardial infarction (MI).
Adverse Effects
Hemorrhage.
All the drugs in this chapter increase the risk for patient bleeding. Bleeding develops in about 10% of patients and is the principal complication of treatment. Hemorrhage can occur at any site and may be fatal. Patients should be monitored closely for signs of blood loss. These include reduced blood pressure, increased heart rate, bruises, petechiae, hematomas, red or black stools, cloudy or discolored urine, pelvic pain (suggesting ovarian hemorrhage), headache or faintness (suggesting cerebral hemorrhage), and lumbar pain (suggesting adrenal hemorrhage). If bleeding develops, heparin should be withdrawn.
The risk for hemorrhage can be decreased in several ways. First, dosage should be carefully controlled so that the activated partial thromboplastin time (see later) does not exceed 2 times the control value. In addition, candidates for heparin therapy should be screened for risk factors (see “Warnings and Contraindications”). Finally, antiplatelet drugs (e.g., aspirin, clopidogrel) should be avoided.
Risk for hematoma is increased by the following:
• Use of an indwelling epidural catheter
• Use of other anticoagulants (e.g., warfarin, dabigatran)
• Use of antiplatelet drugs (e.g., aspirin, clopidogrel)
• History of traumatic or repeated epidural or spinal puncture
• History of spinal deformity, spinal injury, or spinal surgery
Patients should be monitored for signs and symptoms of neurologic impairment. If impairment develops, immediate intervention is needed.
Heparin-Induced Thrombocytopenia (HIT).
This is a potentially fatal immune-mediated disorder characterized by reduced platelet counts (thrombocytopenia) and a seemingly paradoxical increase in thrombotic events. The underlying cause is development of antibodies against heparin–platelet protein complexes. These antibodies activate platelets and damage the vascular endothelium, thereby promoting both thrombosis and a rapid loss of circulating platelets. Thrombus formation poses a risk for DVT, PE, cerebral thrombosis, and MI. Ischemic injury secondary to thrombosis in the limbs may require amputation of an arm or leg. Coronary thrombosis can be fatal. The primary treatment for HIT is discontinuation of heparin and, if anticoagulation is still needed, substitution of a nonheparin anticoagulant (e.g., argatroban). The incidence of HIT is between 0.2% and 5% among patients who receive heparin for more than 4 days.
HIT should be suspected whenever platelet counts fall significantly or when thrombosis develops despite adequate anticoagulation. Accordingly, to reduce the risk for HIT, patients should be monitored for signs of thrombosis and for reductions in platelets. Platelet counts should be determined frequently (2–3 times a week) during the first 3 weeks of heparin use and monthly thereafter. If severe thrombocytopenia develops (platelet count below 100,000/mm3), heparin should be discontinued.
Hypersensitivity Reactions.
Because commercial heparin is extracted from animal tissues, these preparations may be contaminated with antigens that can promote allergy. Possible allergic responses include chills, fever, and urticaria. Anaphylactic reactions are rare.
Other Adverse Effects.
SubQ dosing may produce local irritation and hematoma. Vasospastic reactions that persist for several hours may develop after 1 or more weeks of treatment. Long-term, high-dose therapy may cause osteoporosis.
Warnings and Contraindications
Warnings.
Heparin must be used with extreme caution in all patients who have a high likelihood of bleeding. Among these are individuals with hemophilia, increased capillary permeability, dissecting aneurysm, peptic ulcer disease, severe hypertension, or threatened abortion. Heparin must also be used cautiously in patients with severe disease of the liver or kidneys.
Contraindications.
Heparin is contraindicated for patients with thrombocytopenia and uncontrollable bleeding. In addition, heparin should be avoided both during and immediately after surgery of the eye, brain, or spinal cord. Lumbar puncture and regional anesthesia are additional contraindications.
Drug Interactions
In heparin-treated patients, platelet aggregation is the major remaining defense against hemorrhage. Aspirin and other drugs that depress platelet function or affect coagulation will weaken this defense and hence must be employed with caution.
Laboratory Monitoring
The objective of anticoagulant therapy is to reduce blood coagulability to a level that is low enough to prevent thrombosis but not so low as to promote spontaneous bleeding. Because heparin levels can be highly variable, achieving this goal is difficult and requires careful control of dosage based on frequent tests of coagulation. The laboratory test employed most commonly is the activated partial thromboplastin time (aPTT). The normal value for the aPTT is 40 seconds. At therapeutic levels, heparin increases the aPTT by a factor of 1.5 to 2, making the aPTT 60 to 80 seconds. Because heparin has a rapid onset and brief duration, if an aPTT value falls outside the therapeutic range, coagulability can be quickly corrected through an adjustment in dosage: if the aPTT is too long (more than 80 seconds), the dosage should be lowered; conversely, if the aPTT is too short (less than 60 seconds), the dosage should be increased. Measurements of aPTTs should be made frequently (every 4–6 hours) during the initial phase of therapy. When an effective dosage has been established, measuring aPTT once a day will suffice.
Prescription and Preparations
Prescription.
Heparin is prescribed in units, not in milligrams. The heparin unit is an index of anticoagulant activity. Heparin dosage is titrated on the basis of laboratory monitoring, and hence dosage can be adjusted as needed based on test results.
Preparations.
Heparin sodium is supplied in single-dose vials; multidose vials; and unit-dose, preloaded syringes that have their own needles. Concentrations range from 1000 to 20,000 units/mL.
Dosage and Administration
General Considerations.
Heparin is administered by injection only. Two routes are employed: intravenous (either intermittent or continuous) and subcutaneous. Intramuscular injection causes hematoma and must not be done. Heparin is not administered orally because heparin is too large and too polar to permit intestinal absorption.
Dosage varies by indication. Postoperative prophylaxis of thrombosis, for example, requires relatively small doses. In other situations, such as open heart surgery, much larger doses are needed. The dosages given here are for “general anticoagulant therapy.” As a rule, the aPTT should be employed as a guideline for dosage titration; increases in the aPTT of 1.5- to 2-fold are therapeutic. Because heparin is formulated in widely varying concentrations, you must read the label carefully to ensure that dosing is correct.
PATIENT-CENTERED CARE ACROSS THE LIFE SPAN
Anticoagulants
| Life Stage | Patient Care Concerns |
| Infants | Heparin is commonly used in infants needing anticoagulation. Argatroban has been used successfully in infants with HIT. Warfarin is also administered to infants. |
| Children/adolescents | Many anticoagulants can be used safely in children, just in smaller doses. Side-effect profiles are similar to those of adults. |
| Pregnant women | Warfarin is classified in FDA Pregnancy Risk Category X and is contraindicated in pregnancy. LMW heparins and unfractionated heparin are commonly used in pregnancy. In pregnant women with HIT, argatroban is a safe alternative. |
| Breastfeeding women | Data are lacking regarding safety of these medications in breastfeeding. Warfarin and heparin are both safe to use. |
| Older adults | Atrial fibrillation becomes more common with age. In older adults, benefit must outweigh risk for bleeding secondary to falls, decreased renal function, or polypharmacy. |
Low-Molecular-Weight Heparins
Group Properties
LMW heparins are simply heparin preparations composed of molecules that are shorter than those found in unfractionated heparin. LMW heparins are as effective as unfractionated heparin and are easier to use because they can be given using a fixed dosage and don’t require aPTT monitoring. As a result, LMW heparins can be used at home, whereas unfractionated heparin must be given in a hospital when administering intravenously. Because of these advantages, LMW heparins are now considered first-line therapy for prevention and treatment of DVT. In the United States two LMW heparins are available: enoxaparin [Lovenox] and dalteparin [Fragmin]. Differences between LMW heparins and unfractionated heparin are shown in Table 44.2.
Production.
LMW heparins are made by depolymerizing unfractionated heparin (i.e., breaking unfractionated heparin into smaller pieces). Molecular weights in LMW preparations range between 1000 and 9000, with a mean of 4000 to 5000. In comparison, molecular weights in unfractionated heparin range between 3000 and 30,000, with a mean of 12,000 to 15,000.
Mechanism of Action.
Anticoagulant activity of LMW heparin is mediated by the same active pentasaccharide sequence that mediates anticoagulant action of unfractionated heparin. However, because LMW heparin molecules are short, they do not have quite the same effect as unfractionated heparin. Specifically, whereas unfractionated heparin is equally good at inactivating factor Xa and thrombin, LMW heparins preferentially inactivate factor Xa, being much less able to inactivate thrombin. Why the difference? To inactivate thrombin, a heparin chain must not only contain the pentasaccharide sequence that activates antithrombin but must also be long enough to provide a binding site for thrombin. This binding site is necessary because inactivation of thrombin requires simultaneous binding of thrombin with heparin and antithrombin (see Fig. 44.3). In contrast to unfractionated heparin chains, most (but not all) LMW heparin chains are too short to allow thrombin binding, and hence LMW heparins are less able to inactivate thrombin.
Therapeutic Use.
LMW heparins are approved for (1) prevention of DVT after abdominal surgery, hip replacement surgery, or knee replacement surgery; (2) treatment of established DVT, with or without PE; and (3) prevention of ischemic complications in patients with unstable angina, non–Q-wave MI, and ST-elevation MI (STEMI). In addition, these drugs have been used extensively off-label to prevent DVT after general surgery and in patients with multiple trauma and acute spinal injury. When used for prophylaxis or treatment of DVT, LMW heparins are at least as effective as unfractionated heparin, and possibly more effective.
Pharmacokinetics.
Compared with unfractionated heparin, LMW heparins have higher bioavailability and longer half-lives. Bioavailability is higher because LMW heparins do not undergo nonspecific binding to proteins and tissues and hence are more available for anticoagulant effects. Half-lives are prolonged (up to 6 times longer than that of unfractionated heparin) because LMW heparins undergo less binding to macrophages and hence undergo slower clearance by the liver. Because of increased bioavailability, plasma levels of LMW heparin are highly predictable. As a result, these drugs can be given using a fixed dosage, with no need for routine monitoring of coagulation. Because of their long half-lives, LMW heparins can be given just once or twice a day.
Administration, Dosing, and Monitoring.
All LMW heparins are administered by subQ injection. Dosage is sometimes based on body weight, depending on indication. Because plasma levels of LMW heparins are predictable for any given dose, these drugs can be employed using a fixed dosage without laboratory monitoring. This contrasts with unfractionated heparin, which requires dosage adjustments on the basis of aPTT measurements. Because LMW heparins have an extended half-life, dosing can be done once or twice daily. For prophylaxis of DVT, dosing is begun in the perioperative period and continued 5 to 10 days.
Adverse Effects and Interactions.
Bleeding is the major adverse effect. However, the incidence of bleeding complications is less than with unfractionated heparin. Despite the potential for bleeding, LMW heparins are considered safe for outpatient use. Like unfractionated heparin, LMW heparins can cause immune-mediated thrombocytopenia. As with unfractionated heparin, overdose with LMW heparins can be treated with protamine sulfate.
Like unfractionated heparin, LMW heparins can cause severe neurologic injury, including permanent paralysis, when given to patients undergoing spinal puncture or spinal or epidural anesthesia. The risk for serious harm is increased by concurrent use of antiplatelet drugs (e.g., aspirin, clopidogrel) or anticoagulants (e.g., warfarin, dabigatran). Patients should be monitored closely for signs of neurologic impairment.
Cost.
LMW heparins cost more than unfractionated heparin (e.g., about $63/day for dalteparin vs. $8/day for unfractionated heparin). However, because LMW heparins can be used at home and don’t require aPTT monitoring, the overall cost of treatment is lower than with unfractionated heparin.
Individual Preparations
In the United States two LMW heparins are available: enoxaparin and dalteparin. Additional LMW heparins are available in other countries. Each preparation is unique, so clinical experience with one may not apply fully to the other.
Enoxaparin.
Enoxaparin is approved for prevention of DVT after hip and knee replacement surgery or abdominal surgery in patients considered at high risk for thromboembolic complications (e.g., obese patients, those older than 40 years, and those with malignancy or a history of DVT or PE). The drug is also approved for preventing ischemic complications in patients with unstable angina, non–Q-wave MI, or STEMI.
Administration and Dosage.
Enoxaparin is administered by deep subQ injection. For patients with normal renal function (or moderate renal impairment), dosages are as follows:
• Prevention of DVT after hip or knee replacement surgery—30 mg every 12 hours starting 12 to 24 hours after surgery and continuing 7 to 10 days.
• Prevention of DVT after abdominal surgery—40 mg once daily, beginning 2 hours before surgery and continuing 7 to 10 days.
• Treatment of established DVT—1 mg/kg every 12 hours
• Patients with unstable angina or non–Q-wave MI—1 mg/kg every 12 hours (in conjunction with oral aspirin, 100–325 mg once daily) for 2 to 8 days.
• Patients with acute STEMI—30 mg/kg by IV bolus plus 1 mg/kg subQ, followed by 1 mg/kg subQ every 12 hours for up to 8 days.
For patients with severe renal impairment, dosage should be reduced.
Dalteparin.
Approved indications for dalteparin are prevention of DVT after hip replacement surgery or abdominal surgery in patients considered at high risk for thromboembolic complications, prevention of ischemic complications in patients with unstable angina or non–Q-wave MI, and management of symptomatic venous thromboembolism (VTE). Administration is by deep subQ injection. Dosages are as follows:
• Prevention of DVT after hip replacement surgery—2500 units 1 or 2 hours before surgery, 2500 units that evening (at least 6 hours after the first dose), and then 5000 units once daily for 5 to 10 days.
• Prevention of DVT after abdominal surgery—2500 units once daily for 5 to 10 days, starting 1 to 2 hours before surgery. In high-risk patients, this dose is increased to 5000 units once daily, starting the night before surgery.
• Patients with unstable angina or non–Q-wave MI—120 units/kg (but not more than 10,000 units total) every 12 hours for 5 to 8 days. Concurrent therapy with aspirin (75–165 mg/day) is required.
• Patients with symptomatic VTE—200 units/kg (but not more than 18,000 units total) once daily for 1 month, then 150 units/kg (but not more than 18,000 units total) once daily for months 2 through 6.
Fondaparinux
Actions
Fondaparinux [Arixtra] is a synthetic, subQ anticoagulant that enhances the activity of antithrombin, to cause selective inhibition of factor Xa. The result is reduced production of thrombin and hence reduced coagulation. Note that fondaparinux differs from the heparin preparations, which cause inactivation of thrombin as well as factor Xa.
Fondaparinux is closely related in structure and function to heparin and the LMW heparins. Structurally, fondaparinux is a pentasaccharide identical to the antithrombin-binding region of the heparins. Hence, like the heparins, fondaparinux is able to induce a conformational change in antithrombin, thereby increasing antithrombin’s activity—but only against factor Xa, not against thrombin. Why is fondaparinux selective for factor Xa? Because the drug is quite small—even smaller than the LMW heparins. As a result, it is too small to form a complex with both antithrombin and thrombin, and hence cannot reduce thrombin activity (see Fig. 44.3).
Fondaparinux has no effect on prothrombin time, aPTT, bleeding time, or platelet aggregation.
Therapeutic Use
Fondaparinux is approved for (1) preventing DVT after hip fracture surgery, hip replacement surgery, knee replacement surgery, or abdominal surgery; (2) treating acute PE (in conjunction with warfarin); and (3) treating acute DVT (in conjunction with warfarin). The drug is somewhat more effective than enoxaparin (an LMW heparin) at preventing DVT but may cause slightly more bleeding. Anticoagulation may persist for 2 to 4 days after the last dose. Fondaparinux is administered using a fixed dosage and does not require routine laboratory monitoring.
Pharmacokinetics
Fondaparinux is administered by subQ injection. Bioavailability is 100%. Plasma levels peak 2 hours after dosing. The drug is eliminated by the kidneys with a half-life of 17 to 21 hours. The half-life is increased in patients with renal impairment.
Adverse Effects
As with other anticoagulants, bleeding is the biggest concern. The risk is increased by advancing age and renal impairment. Fondaparinux should be used with caution in patients with moderate renal impairment, defined as creatinine clearance (CrCl) of 30 to 50 mL/minute, and avoided in patients with severe renal impairment, defined as CrCl below 30 mL/minute. The drug should also be avoided for prophylactic use in patients weighing less than 50 kg because low body weight increases bleeding risk. After surgery, at least 6 hours should elapse before starting fondaparinux. Aspirin and other drugs that interfere with hemostasis should be used with caution. In contrast to overdose with heparin or LMW heparins, overdose with fondaparinux cannot be treated with protamine sulfate.
Fondaparinux does not promote immune-mediated HIT, although it still can lower platelet counts. During clinical trials, thrombocytopenia developed in 3% of patients. Platelet counts should be monitored, and if they fall below 100,000/mm3, fondaparinux should be discontinued.
In patients undergoing anesthesia using an epidural or spinal catheter, fondaparinux (as well as other anticoagulants) can cause spinal or epidural hematoma, which can result in permanent paralysis. However, in clinical trials, when fondaparinux was administered no sooner than 2 hours after catheter removal, no hematomas were reported.
Preparations, Dosage, and Administration
Fondaparinux [Arixtra] is available in single-dose, prefilled syringes (2.5, 5, 7.5, and 10 mg). Dosing is done once a day by subQ injection.
For prevention of DVT, the recommended dosage is 2.5 mg once a day, starting 6 to 8 hours after surgery. The usual duration is 5 to 9 days.
For treatment of acute DVT or acute PE, dosage is based on body weight as follows: for patients under 50 kg, 5 mg once daily; for patients 50 to 100 kg, 7.5 mg once daily, and for patients over 100 kg, 10 mg once daily. The usual duration is 5 to 9 days when overlapping with warfarin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Black Box Warning: Spinal or Epidural Hematoma
Black Box Warning: Spinal or Epidural Hematoma