59
Antibodies
CHAPTER CONTENTS
INTRODUCTION
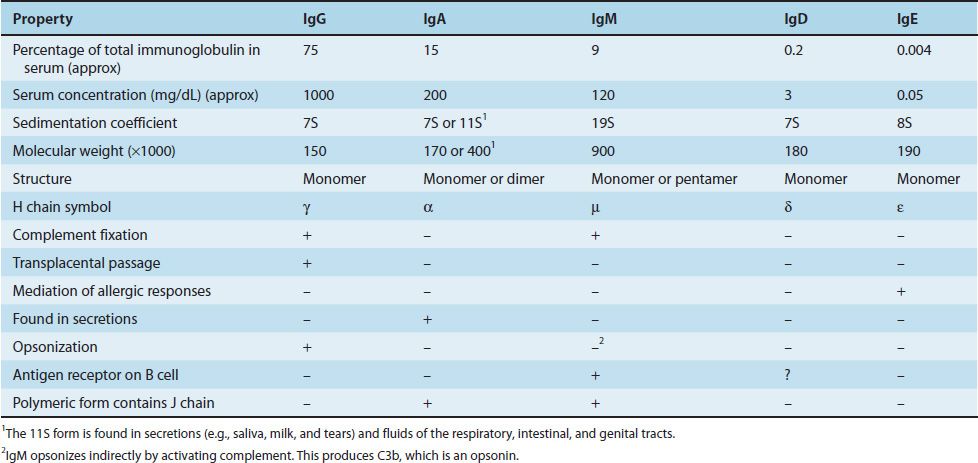
Antibodies are globulin proteins (immunoglobulins [Ig]) that react specifically with the antigen that stimulated their production. They make up about 20% of the protein in blood plasma. Blood contains three types of globulins, alpha, beta, and gamma, based on their electrophoretic migration rate. Antibodies are gamma globulins. There are five classes of antibodies: IgG, IgM, IgA, IgD, and IgE. Antibodies are subdivided into these five classes based on differences in their heavy chains.
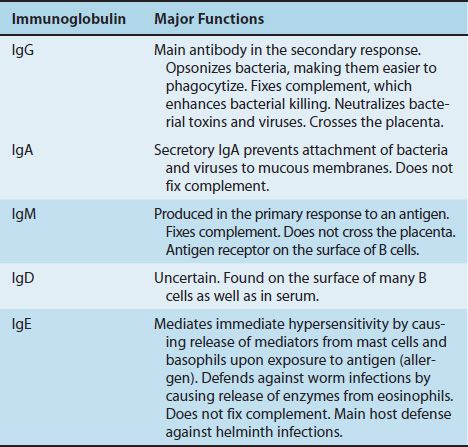
The most important functions of antibodies are to neutralize toxins and viruses, to opsonize microbes so they are more easily phagocytosed, to activate complement, and to prevent the attachment of microbes to mucosal surfaces. The specific antibody classes that mediate these functions are described in Table 59–1. In addition to these functions, antibodies have a catalytic (enzymatic) capability that is described in a separate section at the end of this chapter.
MONOCLONAL ANTIBODIES
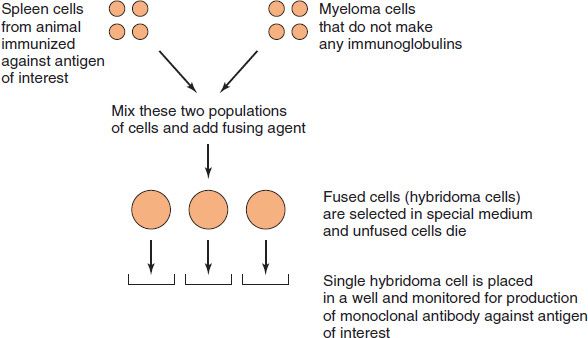
Antibodies that arise in an animal in response to typical antigens are heterogeneous, because they are formed by several different clones of plasma cells (i.e., they are polyclonal). Antibodies that arise from a single clone of cells (e.g., in a plasma cell tumor [myeloma])1 are homogeneous (i.e., they are monoclonal).
Monoclonal antibodies also can be made in the laboratory by fusing a myeloma cell with an antibody-producing cell (Figure 59–1; also see box “Hybridomas & Monoclonal Antibodies”). Such hybridomas produce virtually unlimited quantities of monoclonal antibodies that are useful in diagnostic tests and in research (see box “Hybridomas & Monoclonal Antibodies”).
IMMUNOGLOBULIN STRUCTURE
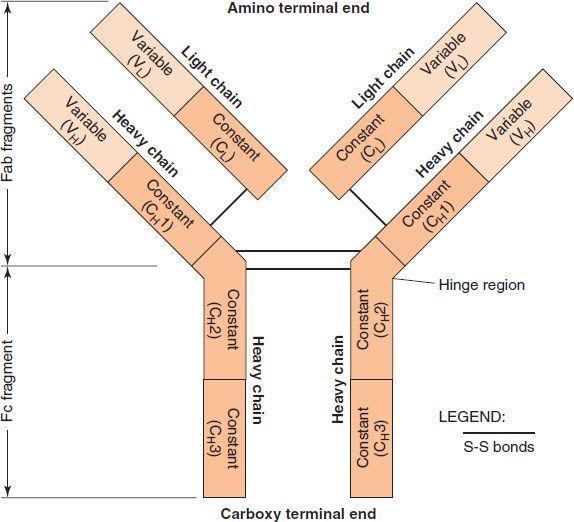
Immunoglobulins are glycoproteins made up of light (L) and heavy (H) polypeptide chains. The terms light and heavy refer to molecular weight; light chains have a molecular weight of about 25,000, whereas heavy chains have a molecular weight of 50,000 to 70,000. The simplest antibody molecule has a Y shape (Figure 59–2) and consists of four polypeptide chains: two H chains and two L chains. The four chains are linked by disulfide bonds. An individual antibody molecule always consists of identical H chains and identical L chains. This is primarily the result of two phenomena: allelic exclusion (see page 514) and regulation within the B cell, which ensure the synthesis of either kappa (κ) or lambda (λ) L chains, but not both.
FIGURE 59–2 Structure of immunoglobulin G (IgG). The Y-shaped IgG molecule consists of two light chains and two heavy chains. Each light chain consists of a variable region and a constant region. Each heavy chain consists of a variable region and a constant region that is divided into three domains: CH1, CH2, and CH3. The CH2 domain contains the complement-binding site, and the CH3 domain is the site of attachment of IgG to receptors on neutrophils and macrophages. The antigen-binding site is formed by the variable regions of both the light and heavy chains. The specificity of the antigen-binding site is a function of the amino acid sequence of the hypervariable regions (see Figure 59–3). (Modified and reproduced with permission from Brooks GF et al. Medical Microbiology. 20th ed. Originally published by Appleton & Lange. Copyright 1995 McGraw-Hill.)
L and H chains are subdivided into variable and constant regions. The regions are composed of three-dimensionally folded, repeating segments called domains. An L chain consists of one variable (VL) and one constant (CL) domain. Most H chains consist of one variable (VH) and three constant (CH) domains. (IgG and IgA have three CH domains, whereas IgM and IgE have four.) Each domain is approximately 110 amino acids long. The variable regions of both the light and heavy chain are responsible for antigen-binding, whereas the constant region of the heavy chain is responsible for various biologic functions (e.g., complement activation and binding to cell surface receptors). The complement binding site is in the CH2 domain. The constant region of the light chain has no known biologic function.
HYBRIDOMAS & MONOCLONAL ANTIBODIE
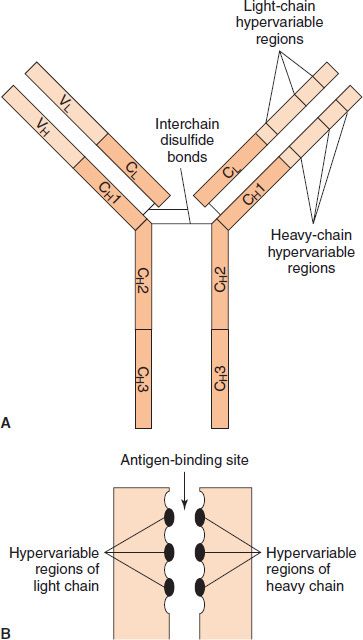
The variable regions of both L and H chains have three extremely variable (hypervariable) amino acid sequences at the amino-terminal end that form the antigen-binding site (Figure 59–3). Only 5 to 10 amino acids in each hypervariable region form the antigen-binding site. Antigen–antibody binding involves electrostatic and van der Waals’ forces and hydrogen and hydrophobic bonds rather than covalent bonds. The remarkable specificity of antibodies is due to these hypervariable regions (see the discussion of idiotypes on page 512).
FIGURE 59–3 The antigen-binding site is formed by the hypervariable regions. A: Hypervariable regions on immunoglobulin G (IgG). B: Magnified view of antigen-binding site. (Modified and reproduced with permission from Stites DP, Terr A, Parslow T, eds. Basic & Clinical Immunology. 8th ed. Originally published by Appleton & Lange. Copyright 1994 McGraw-Hill.)
L chains belong to one of two types, κ (kappa) or λ (lambda), on the basis of amino acid differences in their constant regions. Both types occur in all classes of immunoglobulins (IgG, IgM, etc.), but any one immunoglobulin molecule contains only one type of L chain.2
The amino-terminal portion of each L chain participates in the antigen-binding site. H chains are distinct for each of the five immunoglobulin classes and are designated γ, α, μ, ε, and δ (Table 59–2). The amino-terminal portion of each H chain participates in the antigen-binding site; the carboxy terminal forms the Fc fragment, which has the biologic activities described earlier and in Table 59–2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree