DRUG CLASSES
Penicillins
• Natural penicillins
• Aminopenicillins
• Extended-spectrum penicillins
Cephalosporins
• First generation
• Second generation
• Third generation
• Fourth generation
Carbapenems
PHARMACOLOGY IN PRACTICE

Alfredo Garcia is seen in the outpatient clinic for an upper respiratory infection. The primary health care provider prescribes a cephalosporin and asks you to give the patient instructions for taking the drug. You note that Mr. Garcia appears to understand very little English.
The cellular content in a human being is contained by a cell membrane. Unlike the human cell, a bacterial cell has a wall, not a membrane. Penicillins, cephalosporins, carbapenems, and vancomycin all inhibit bacterial cell wall synthesis (growth and repair).
Enzymes known as penicillin-binding proteins (PBPs) are involved in bacterial cell wall synthesis and cell division. Antibiotics, which interfere with these processes, inhibit cell wall synthesis, causing rapid destruction of the bacterial cell. When penicillin was first used, these drugs worked very well because bacteria have a receptor on the cell wall that attracts the penicillin molecule. That is, when the drug attaches to the cell, a portion of the drug molecule (the beta (b)-lactam ring) breaks the cell wall and the cell dies (bactericidal action). Now, however, after many years of use of the penicillins, drug-resistant strains of microorganisms developed, making the penicillins less effective than some of the new antibiotics in treating a broad range of infections.
Cephalosporins are structurally and chemically related to penicillin. The cephalosporins are a valuable group of drugs that are effective in the treatment of infection with almost all of the strains of bacteria affected by the penicillins, as well as some strains of bacteria that have become resistant to penicillin. Carbapenems are a relatively new class of bacteriocidal drugs that have the largest spectrum of any antibiotic (Fig. 7.1).
Identifying the Appropriate Anti-Infective
Selection of Drugs
After a culture and sensitivity report is received, the strain of microorganisms causing the infection is known as well as which antibiotics will or will not kill the microorganisms (see Chapter 6). The primary health care provider then selects the antibiotic to which the microorganism is sensitive, because that is the antibiotic that will be effective in the treatment of the infection.
Drugs have bactericidal action provided there is an adequate concentration of the drug in the body. The concentration of any drug in the body is referred to as the blood level. An inadequate concentration (or inadequate blood level) of an antibiotic may produce bacteriostatic activity, which may or may not control the infection.
Resistance to Drugs
Bacterial resistance is the ability of bacteria to produce substances that inactivate or destroy the antibiotic. Because bacteria have this ability, many more drugs have been developed in addition to the sulfonamides and penicillins to fight bacterial infections. They include cephalosporins, the tetracycline group, and various other drugs.
When antibiotics are used by one person over time, or by a group of people who live in close proximity (as in a long-term care facility), drug resistance becomes an issue. Some bacteria may be naturally resistant to an antibiotic, or they may acquire a resistance to the drug. When the susceptible bacteria are destroyed, what remains are the resistant bacteria. As a result, strains of drug-resistant bacteria multiply. These can range from the penicillinase enzyme–producing bacteria to methicillin-resistant Staphylococcus aureus (MRSA).
MRSA is a type of bacteria that is resistant to certain antibiotics. These antibiotics include methicillin and other, more common antibiotics, such as oxacillin, penicillin, and amoxicillin. According to the Centers for Disease Control and Prevention (CDC), staphylococcal infections, including MRSA, occur most frequently among persons who are in hospitals and health care facilities (such as skilled nursing facilities and dialysis centers) and who have weakened immune systems. In recent years, outbreaks in the community have heightened the public’s awareness of MRSA. Also emerging is a new resistance associated with bacteria that have both natural and acquired resistance. An example is vancomycin-resistant enterococci (VRE). This drug resistance is affecting severely ill, immunocompromised patients in intensive care, transplant, and some cancer treatment units.
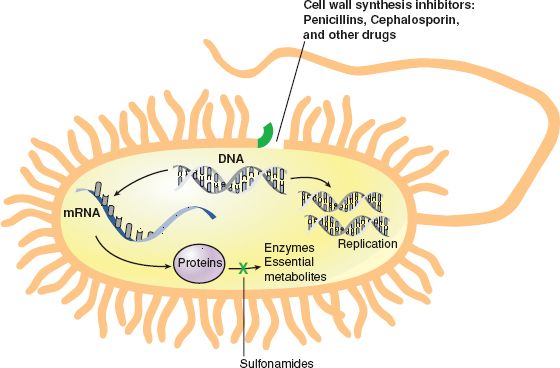
Figure 7.1 Cell wall synthesis inhibitors: penicillins, cephalosporin, and other drugs.
Patient education to increase behaviors that will prevent or diminish resistance is important when antibiotics are prescribed (see Patient Teaching for Improved Patient Outcomes: Preventing Anti-Infective Resistance at the end of this chapter).
PENICILLINS
The antibacterial properties of natural penicillins were discovered in 1928 and used clinically to treat infections by 1941. Although the sulfonamides were the first anti-infectives, the natural penicillins were the first large-scale antibiotics used to combat infection. Used for more than 70 years, the penicillins are still an important and effective group of antibiotics for the treatment of susceptible pathogens (disease-causing microorganisms).
Actions
There are four groups of penicillins: (1) natural penicillins, (2) penicillinase-resistant penicillins, (3) aminopenicillins, and (4) extended-spectrum penicillins, All work to inhibit the integrity of the bacterial cell wall. See the Summary Drug Table: Antibacterial Drugs That Inhibit Cell Wall Synthesis for a more complete listing of the penicillins.
The natural penicillins have a fairly narrow spectrum of activity, which means that they are effective against only a few strains of bacteria. Newer, chemically modified aminopenicillins were developed to combat this problem. Because of their chemical modifications, they are more slowly excreted by the kidneys and thus have a somewhat wider spectrum of antibacterial activity.
Various types of bacterial resistance have developed against the penicillins. One example of bacterial resistance is the ability of certain bacteria to produce penicillinase, an enzyme that inactivates penicillin. The penicillinase-resistant penicillins were developed to combat this problem.
Certain bacteria have developed the ability to produce enzymes called β-lactamases, which are able to destroy the β-lactam ring of the drug. Fortunately, chemicals were discovered that inhibit the activity of these enzymes. Penicillin–β-lactamase inhibitor combinations are a type of penicillin with a wider spectrum of antibacterial activity. Examples of these β-lactamase inhibitors are clavulanic acid, sulbactam, and tazobactam. When these chemicals are used alone, they have little antimicrobial activity. However, when combined with certain penicillins, they extend the spectrum of the penicillin’s antibacterial activity. The β-lactamase inhibitors bind with the penicillin and protect the penicillin from destruction. Examples of combinations of penicillins with β-lactamase inhibitors are given in Display 7.1; also see the Summary Drug Table: Antibacterial Drugs That Inhibit Cell Wall Synthesis for more information on these combinations.
Display 7.1 Penicillin– β-Lactamase Inhibitor Combinations
• Augmentin—combination of amoxicillin and clavulanic acid
• Timentin—combination of ticarcillin and clavulanic acid
• Unasyn—combination of ampicillin and sulbactam
• Zosyn—combination of piperacillin and tazobactam
Extended-spectrum penicillins are effective against an even wider range of bacteria than the broad-spectrum penicillins. These penicillins are used to destroy bacteria such as Pseudomonas.
Uses
Infectious Disease
The natural and semisynthetic penicillins are used in the treatment of moderate to mildly severe bacterial infections. Penicillins may be used to treat infections such as:
• Urinary tract infections (UTIs)
• Septicemia
• Meningitis
• Intra-abdominal infections
• Sexually transmitted infections (syphilis)
• Pneumonia and other respiratory infections
Examples of infectious microorganisms (bacteria) that may respond to penicillin therapy include pneumococci and group A β-hemolytic streptococci. Because of the increasing resistance of staphylococci to penicillin G, the penicillinase-resistant penicillins are used as initial therapy for any suspected staphylococcal infection until culture and sensitivity results are known.
Prophylaxis
Because penicillin targets bacterial cells, it is of no value in treating viral or fungal infections. However, the primary health care provider occasionally prescribes penicillin as prophylaxis (prevention) against a potential secondary bacterial infection that can occur in a patient with a viral infection. In these situations the viral infection has weakened the body’s defenses and the person is susceptible to other infections, particularly a bacterial infection. Penicillin also may be prescribed as prophylaxis for a potential infection in high-risk individuals, such as those with a history of rheumatic fever. Penicillin is taken several hours or in some instances days before and after an operative procedure, such as dental, oral, or upper respiratory tract procedures, that can result in bacteria entering the bloodstream. Taking penicillin before and after the procedure will usually prevent a bacterial infection in high-risk patients. Penicillin also may be given prophylactically on a continuing basis to those with rheumatic fever or chronic ear infections.
Adverse Reactions
Gastrointestinal System Reactions
• Glossitis (inflammation of the tongue) when given orally
• Stomatitis (inflammation of the mouth), dry mouth
• Gastritis
• Nausea, vomiting
• Diarrhea, abdominal pain
Administration route reactions include pain at the injection site when given intramuscularly (IM) and irritation of the vein and phlebitis (inflammation of a vein) when given intravenously (IV).
Hypersensitivity Reactions
A hypersensitivity (or allergic) reaction to a drug occurs in some individuals, especially those with a history of allergy to many substances. Signs and symptoms of a hypersensitivity to penicillin are highlighted in Display 7.2.
An anaphylactic reaction, which is a severe form of hypersensitivity, also can occur (see Chapter 1). Anaphylactic reactions occur more frequently after parenteral administration but can occur with oral use. This reaction is likely to be immediate and severe in susceptible individuals. Signs of anaphylactic reaction or shock include severe hypotension, loss of consciousness, and acute respiratory distress. If not immediately treated, anaphylactic shock can be fatal.
Once an individual is allergic to one penicillin, he or she is usually allergic to all of the penicillins. Those allergic to penicillin also have a higher incidence of allergy to the cephalosporins. Allergy to drugs in the same or related groups is called cross-sensitivity.
Other Reactions
Other adverse reactions associated with penicillin include hematopoietic (blood cell) changes:
• Anemia (low red blood cell count)
• Thrombocytopenia (low platelet count)
• Leukopenia (low white blood cell count)
• Bone marrow depression
Display 7.2 Signs and Symptoms of Hypersensitivity to Penicillin and Other Antibiotics
• Skin rash
• Urticaria (hives)
• Sneezing
• Wheezing
• Pruritus (itching)
• Bronchospasm (spasm of the bronchi)
• Laryngospasm (spasm of the larynx)
• Angioedema (also called angioneurotic edema)—swelling of the skin and mucous membranes, especially around and in the mouth and throat
• Hypotension—can progress to shock
• Signs and symptoms resembling serum sickness—chills, fever, edema, joint and muscle pain, and malaise
Contraindications and Precautions
Penicillins are contraindicated in patients with a history of hypersensitivity to penicillin or the cephalosporins.
Penicillins should be used cautiously in patients with renal disease, asthma, bleeding disorders, gastrointestinal (GI) disease, pregnancy (pregnancy category C) or lactation (may cause diarrhea or candidiasis in the infant), and history of allergies. Any indication of sensitivity is reason for caution.
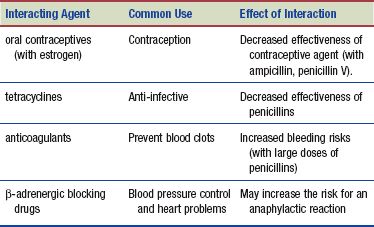
Interactions
The following interactions may occur when a penicillin is administered with another agent.
 HERBAL CONSIDERATIONS
HERBAL CONSIDERATIONS
Goldenseal (Hydrastis canadensis) is an herb found growing in certain areas of the northeastern United States, particularly the Ohio River valley. Goldenseal has been used to wash inflamed or infected eyes and in making yellow dye. There are many more traditional uses of the herb, including as an antiseptic for the skin, as a mouthwash for canker sores, and in the treatment of sinus infections and digestive problems such as peptic ulcers and gastritis. In the 19th century, goldenseal was touted as an “herbal antibiotic” for treating gonorrhea and UTIs. Though used over time by American Indian tribes as an insect repellent, stimulant, and diuretic, there is no scientific evidence to support its benefit for these purposes. Another myth surrounding goldenseal’s use is that taking the herb masks the presence of illicit drugs in the urine. Evidence does support the use of goldenseal to treat diarrhea caused by bacteria or intestinal parasites, such as Giardia. The herb is contraindicated during pregnancy and in patients with hypertension. Adverse reactions are rare when the herb is used as directed. However, this herb should not be taken for more than a few days to 1 week (DerMarderosian, 2003).
CEPHALOSPORINS
The cephalosporins are divided into first-, second-, third-, and fourth-generation drugs. In general, progression from the first-generation to the fourth-generation drugs shows an increase in the sensitivity of gram-negative microorganisms and a decrease in the sensitivity of gram-positive microorganisms. For example, a first-generation cephalosporin would be more useful against gram-positive microorganisms than would a third-generation cephalosporin. This scheme of classification is becoming less clearly defined as newer drugs are introduced. The fourth generation of cephalosporins has a broader spectrum and longer duration of resistance to β-lactamase. These drugs are used to treat urinary tract and skin infections and hospital-acquired pneumonias. Examples of first-, second-, third-, and fourth-generation cephalosporins are listed in Display 7.3. For a more complete listing, see the Summary Drug Table: Antibacterial Drugs That Inhibit Cell Wall Synthesis.
Display 7.3 Examples of First-, Second-, Third-, and Fourth-Generation Cephalosporins
• First generation—cephalexin (Keflex), cefazolin (Ancef)
• Second generation—cefaclor (Raniclor), cefoxitin (Mefoxin), cefuroxime (Zinacef)
• Third generation—cefoperazone (Cefobid), cefotaxime (Claforan), ceftriaxone (Rocephin)
• Fourth generation—cefepime (Maxipime)
Actions
Cephalosporins have a β-lactam ring and target the bacterial cell wall, making it defective and unstable. This action is similar to the action of penicillin. The cephalosporins are usually bactericidal.
Uses
The cephalosporins are used in the treatment of infections caused by bacteria, including:
• Respiratory infections
• Otitis media (ear infection)
• Bone/joint infections
• Genitourinary tract and other infections caused by bacteria
Cephalosporins are used prophylactically to prevent infection when victims are treated following a sexual assault. The most frequent infections diagnosed following an assault include trichomoniasis, bacterial vaginitis, gonorrhea, and chlamydia. A cephalosporin drug is the primary drug in post–sexual assault medication protocols (see Appendix E).
This class of drugs also may be used throughout the perioperative period—that is, during the preoperative, intraoperative, and postoperative periods—to prevent infection in patients having surgery on a contaminated or potentially contaminated area, such as the GI tract or vagina. In some instances, a specific drug may be recommended for postoperative prophylactic use only.
Adverse Reactions
Gastrointestinal System Reactions
• Nausea
• Vomiting
• Diarrhea
Other Reactions
• Headache
• Dizziness
• Malaise
• Heartburn
• Fever
• Nephrotoxicity
• Hypersensitivity (allergic) reactions—may occur with administration of the cephalosporins and may range from mild to life threatening. Mild hypersensitivity reactions include pruritus, urticaria, and skin rashes; the more serious reactions include Stevens-Johnson syndrome and hepatic and renal dysfunction.
• Aplastic anemia (deficient red blood cell production)
• Toxic epidermal necrolysis (death of the epidermal layer of the skin)
 NURSING ALERT
NURSING ALERT
Because of the close relationship of the cephalosporins to penicillin, a patient who is allergic to penicillin also may be allergic to the cephalosporins. Approximately 10% of the people allergic to a penicillin drug are also allergic to a cephalosporin drug.
Administration route reactions include pain, tenderness, and inflammation at the injection site when given IM, and phlebitis or thrombophlebitis along the vein when given IV. Therapy with cephalosporins may result in a bacterial or fungal superinfection. Diarrhea may be an indication of pseudomembranous colitis, which is one type of bacterial superinfection (see Chapter 9).
Contraindications and Precautions
Do not administer cephalosporins if the patient has a history of allergies to cephalosporins.
Cephalosporins should be used cautiously in patients with renal disease, hepatic impairment, bleeding disorder, pregnancy (pregnancy category B), and known penicillin allergy.
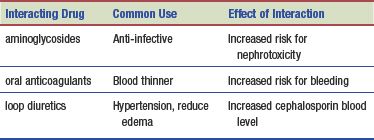
Interactions
The following interactions may occur when a cephalosporin is administered with another agent:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


