18
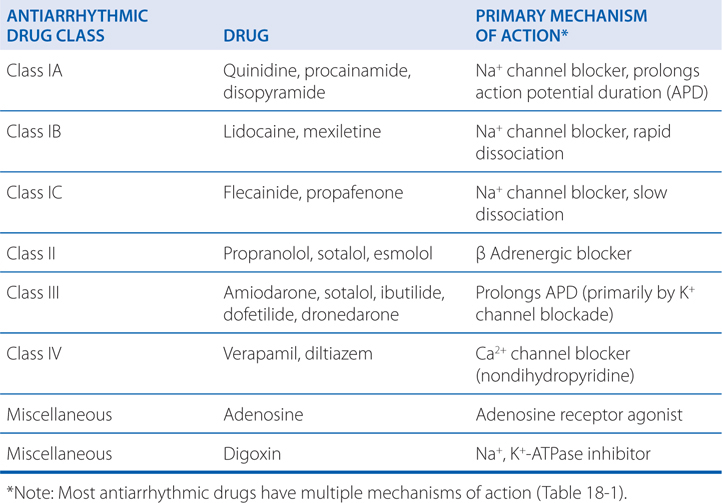
Antiarrhythmic Drugs
This chapter will be most useful after having a basic understanding of the material in Chapter 29, Anti-Arrhythmic Drugs in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In particular, the reader is directed to the following tables, and to animations available in the online version of Goodman & Gilman’s The Pharmacological Basis of Therapeutics:
• Table 29-1 Drug-Induced Cardiac Arrhythmias, which shows drugs known to cause arrhythmias (ie, proarrhythmias), the likely arrhythmogenic mechanism, the treatment, and the clinical features of the proarrhythmia
• Table 29-4 Patient-Specific Anti-arrhythmic Drug Contraindications, which shows conditions and contraindicated drugs
• Several animations in the online version of Goodman & Gilman’s The Pharmacological Basis of Therapeutics illustrate the electrophysiology of cardiac cells and the mechanism of action of antiarrhythmic drugs
LEARNING OBJECTIVES
 Know the principles of cardiac electrophysiology especially the ion channels, exchangers, and pumps that are targets of antiarrhythmic drugs.
Know the principles of cardiac electrophysiology especially the ion channels, exchangers, and pumps that are targets of antiarrhythmic drugs.
 Understand the mechanisms that cause cardiac arrhythmias.
Understand the mechanisms that cause cardiac arrhythmias.
 Know the common and important tachyarrhythmias and their mechanisms.
Know the common and important tachyarrhythmias and their mechanisms.
 Understand the mechanisms and classification of antiarrhythmic drugs.
Understand the mechanisms and classification of antiarrhythmic drugs.
 Know the principles of antiarrhythmic drug pharmacotherapy.
Know the principles of antiarrhythmic drug pharmacotherapy.
 Know the pharmacological, pharmacokinetic, and adverse effects of specific antiarrhythmic agents.
Know the pharmacological, pharmacokinetic, and adverse effects of specific antiarrhythmic agents.
DRUGS IN THIS CHAPTER
• Adenosine (ADENOCARD)
• Amiodarone (CORDARONE)
• Digoxin (LANOXIN; see Chapter 17)
• Diltiazem (CARDIZEM)
• Disopyramide (NORPACE)
• Dofetilide (TIKOSYN)
• Dronedarone (MULTAQ)
• Esmolol (BREVIBLOC)
• Flecainide (TAMBOCOR)
• Ibutilide (CORVERT)
• Lidocaine (XYLOCAINE)
• Mexiletine (MEXITIL)
• Procainamide (PRONESTYL)
• Propafenone (RYTHMOL)
• Propranolol (INDERAL; see Chapter 7)
• Quinidine (QUINIDEX)
• Sotalol (BETAPACE)
• Verapamil (CALAN; see Chapter 16)
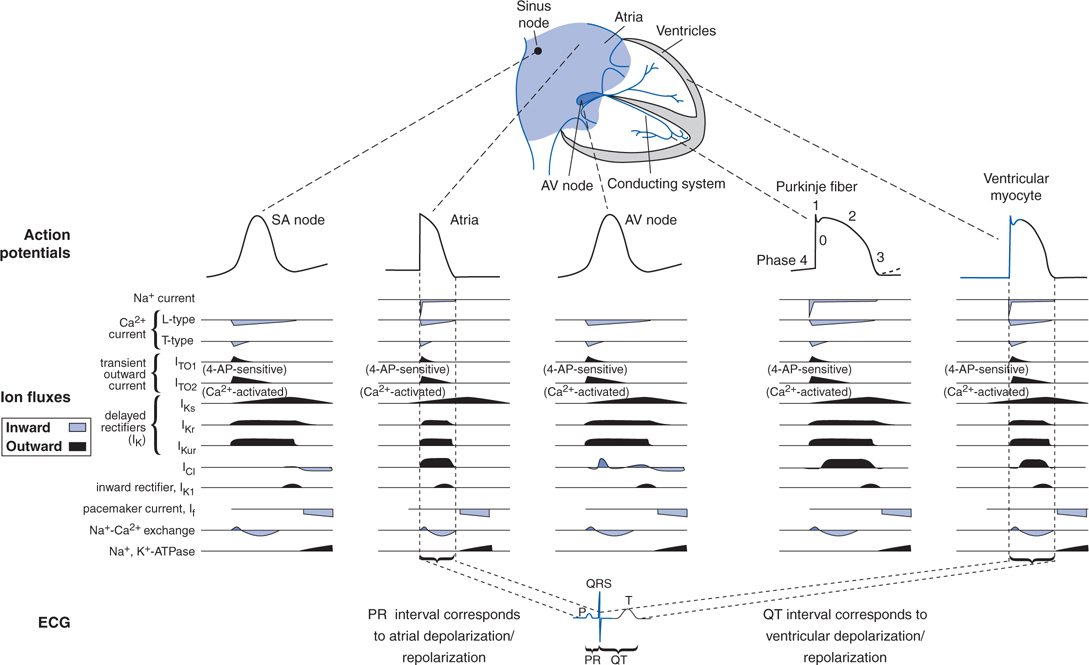
THE CARDIAC ACTION POTENTIAL
• In fast-conducting cardiac myocytes and cells of the conducting system, Phase 0 depolarization is caused by the opening of voltage-gated Na+ channels.
• In slow-conducting cells of the sinoatrial (SA) node and atrioventricular (AV) node, which have relatively few voltage-gated Na+ channels, Phase 0 depolarization is caused by opening of voltage-gated L-type Ca2+ channels.
• Phase 3 repolarization is primarily caused by K+ movement out of the cell through voltage-gated K+ channels.
• Figure 18-1 shows action potentials from different regions of the heart, the ion currents that contribute to these action potentials, and the corresponding effects of the ion currents on the electrocardiogram (ECG).
FIGURE 18-1 Action potentials that occur during normal impulse propagation and the time course of the ion currents that generate them.
MECHANISMS OF ACTION OF DRUGS USED TO TREAT CARDIAC ARRHYTHMIAS
A 53-year-old woman visits the emergency department after losing consciousness while working in her garden. She says she has recently had episodes of dizziness and fainting. Her ECG looks unremarkable except that the QT interval is prolonged.
a. What are the possible causes of her recent fainting spells?
The prolonged QT interval suggests that her fainting spells may be due to torsade de pointes (TdP) (see Side Bar LONG QT AND TORSADE DE POINTES). Because her syncope has started fairly recently, it is likely that the TdP might be due to a drug that prolongs action potential duration. Drugs that can prolong QT include Class IA and Class III antiarrhythmic drugs (see MECHANISMS OF ACTION OF DRUGS USED TO TREAT CARDIAC ARRHYTHMIAS), as well as many other drugs, including antibiotics (erythromycin, sparfloxacin), antipsychotics (chlorpromazine, haloperidol), and antiemetics (domperidone, droperidol). Another possible cause is hypokalemia, especially if she has been taking diuretics that cause loss of K+. She might also have a form of hereditary long QT that has become manifested due to a QT-prolonging drug or hypokalemia. It is important to determine the drugs that this patient is currently taking and whether any of them have been started recently. It would also be useful to know whether there is a family history of sudden cardiac death which might indicate she has a hereditary form of long QT syndrome.
MECHANISMS OF CARDIAC TACHYARRHYTHMIAS
• Three arrhythmogenic mechanisms are thought to underlie most tachyarrhythmias:
▶ Enhanced automaticity resulting from enhanced phase 4 spontaneous depolarization (see Figure 29-10, Goodman & Gilman, 12th Edition)
▶ Triggered automaticity, including abnormal rhythms associated with delayed afterdepolarizations (DADs) resulting from Ca2+ overload, and those associated with early afterdepolarizations (EADs) caused by prolongation of the action potential (see Figure 29-6, Goodman & Gilman, 12th Edition)
▶ Reentry, which involves the formation of a self-perpetuating circuit caused by anisotropic conduction around an anatomic or functional barrier (see Figures 29-7 and 29-8, Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition)
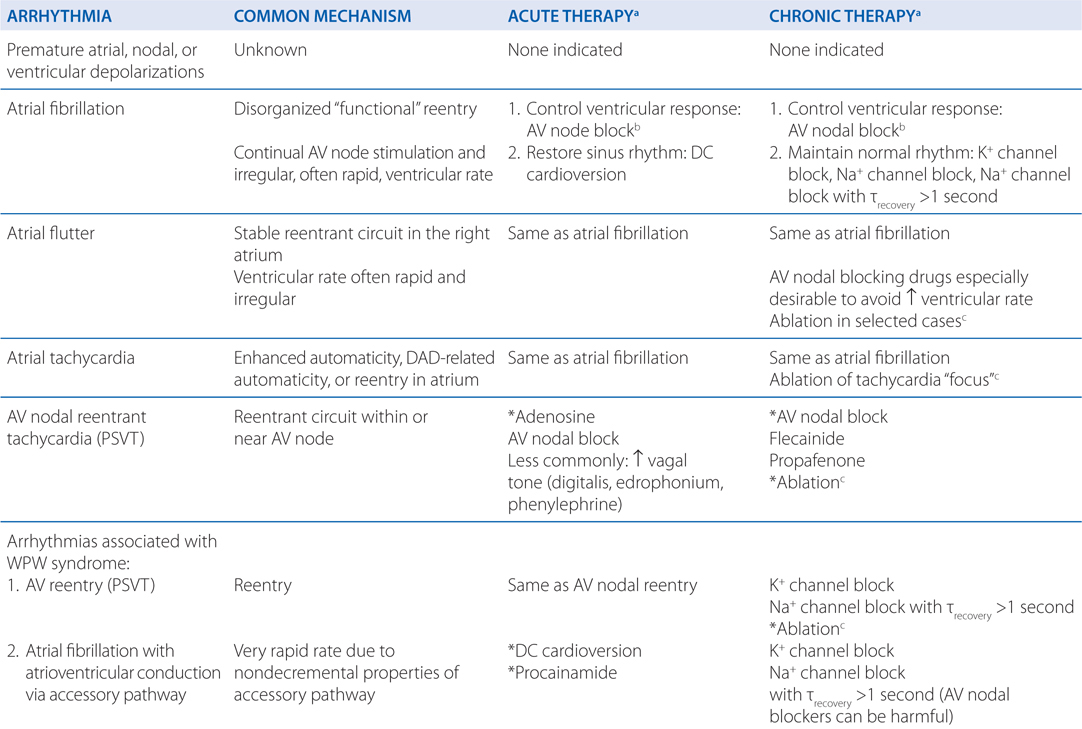
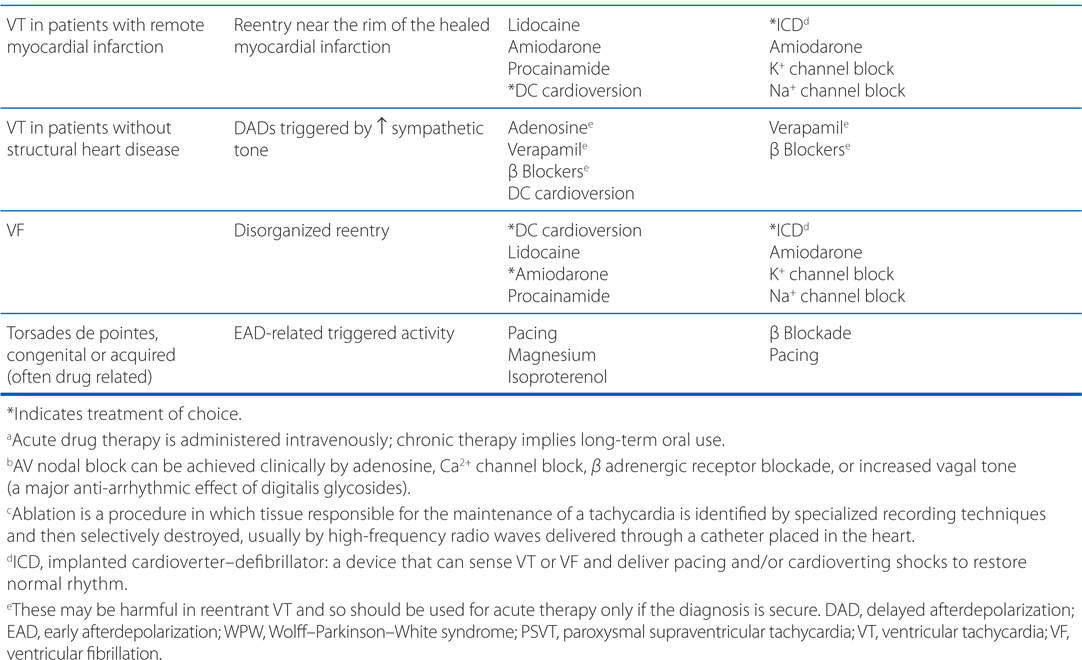
• Understanding the underlying arrhythmogenic mechanism can facilitate the choice of antiarrhythmic drugs (see Table 18-1)
TABLE 18-1 A Mechanistic Approach to Antiarrhythmic Therapy
b. What are your therapeutic options?
If the fainting is thought to be due to TdP, it is important to first identify the cause of this arrhythmia and remove it, if possible. Drugs are the most likely cause so it is important to review the patient’s medications, particularly ones that she has started taking recently. If she is hypokalemic, this should be corrected with supplemental K+. If she is taking a diuretic, her dosing could be reduced or she should be changed to a potassium-sparing diuretic. If the patient is suspected of carrying one of the mutations that results in hereditary long QT syndrome, genetic testing that involves sequencing all of these genes may facilitate appropriate choice of therapy. For instance, patients with hereditary long QT whose TdP is brought on by exercise and activation of the sympathetic nervous system may benefit from β-adrenergic blockers.
LONG QT AND TORSADE DE POINTES
• A prolongation of the QT interval on the ECG is an indication that repolarization of ventricular myocytes is slowed.
• Because the outward repolarizing currents (the delayed rectifier currents) are carried by various voltage-gated K+ channels, drugs that inhibit these channels can prolong QT interval.
• Mutations in genes that regulate these K+ channels can lead to hereditary long QT syndrome (LQT).
• Patients with drug-induced LQT (DILQT) or hereditary LQT are at risk of developing a life-threatening polymorphic ventricular tachycardia known as torsade de pointes (TdP; “twisting of the points” in French) which can progress to ventricular fibrillation.
• TdP is so-named because the QRS complexes progressively increase and decrease in size giving the illusion of a picket fence that is being twisted around a central axis (see Figure 29-9 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition).
• Treatment of patients who develop TdP include removing the underlying cause, if known, and intravenous magnesium sulfate to terminate the arrhythmia.
A 47-year-old man with uncomplicated atrial fibrillation is prescribed diltiazem.
a. What is the mechanistic rationale for using diltiazem to treat atrial fibrillation?
Diltiazem inhibits L-type voltage-gated Ca2+ channels in the SA and AV nodes of the heart. By inhibiting these channels in the AV node, conduction of impulses through the AV node is slowed or blocked. This protects the ventricular rate of depolarization from being controlled by the aberrantly high rate of depolarization of the atria.
b. What effect will diltiazem have on the ECG of this patient?
In a patient with atrial fibrillation, the ventricular rate can be rapid and irregular. This will result in an ECG in which the RR intervals are relatively short and variable. Diltiazem is expected to slow ventricular rate and reduce irregular ventricular depolarizations which will be seen on the ECG as a prolongation of RR intervals and an increase in the regularity of RR intervals. In a patient without atrial fibrillation, diltiazem will increase the PR interval (which reflects the time an impulse takes to travel through the AV node) and increase the RR interval secondary to slowing the spontaneous depolarization of the SA node.
c. What are the other effects and potential risks of using diltiazem?
Because diltiazem blocks L-type Ca2+ channels in the heart and in arterial smooth muscle cells, the drug has negative inotropic effects on the heart and lowers blood pressure because of its vasodilating effects. In patients with poor ventricular function or low blood pressure, this agent could further reduce cardiac output and lower mean arterial pressure.
d. If diltiazem is not well tolerated by this patient, what other classes of drugs might be useful in treating this patient’s atrial fibrillation?
Other classes of drugs that could be used for ventricular rate control include β adrenergic blockers. To treat the arrhythmogenesis of atrial fibrillation, antiarrhythmic drugs that block Na+ channels and/or K+ channels in atrial myocytes might be useful (see Table 18-1).
e. What other class of drugs are indicated in patients with uncomplicated atrial fibrillation?
Patients with atrial fibrillation are at significantly increased risk of stroke and other thromboembolic events due to stasis of blood in the atria. To reduce the risk of blood clots, patients with sustained uncomplicated atrial fibrillation typically take oral anticoagulants such as warfarin, dabigatran, rivaroxaban, or apixaban (see Chapter 19).
A 59-year-old man suddenly loses consciousness in a shopping mall. A security guard at the mall cannot detect a pulse and uses an automated external defibrillator (AED) to resuscitate the man. He regains consciousness and is taken by ambulance to a nearby hospital. In the emergency department waiting room, he loses consciousness again. His ECG shows that he is in ventricular fibrillation.
a. What treatment will this patient receive in the ED?
Ventricular fibrillation is a life-threatening arrhythmia and must be treated aggressively to restore normal sinus rhythm. The patient should be defibrillated and should be administered an intravenous antiarrhythmic drug such as amiodarone, lidocaine, or procainamide to prevent recurrence. Of these 3 drugs, IV amiodarone is the drug of choice for this indication (see Table 18-1).
b. What is the mechanistic rationale for the drug(s) used for the acute treatment of patients in ventricular fibbrillation?
Ventricular fibrillation is characterized by disorganized reentry in the ventricular myocardium. Amiodarone, lidocaine, and procainamide all block the voltage-gated Na+ channel and increase the threshold voltage required to open the channel. Moreover, all 3 agents exhibit state-dependent block of the Na+ channel (Table 18-2), with amiodarone binding the Inactive (I) state, procainamide binding the Open (O) state, and lidocaine binding both the Inactive and Open states (I>O). By blocking the Na+ channel in the Open and Inactive states, the drugs will preferentially block rapidly depolarizing myocytes, leaving those myocytes depolarizing at normal rates relatively unaffected. Thus, any reentrant circuits formed in the ventricles following the electrical defibrillation will be selectively inhibited, thereby preventing recurrence of the tachyarrhythmia. In addition to blocking the Na+ channel, amiodarone and procainamide also have antiarrhythmic effects through their actions on blocking outward K+ currents.
TABLE 18-2 Major Electrophysiologic Actions of Anitarrhythmic Drugs
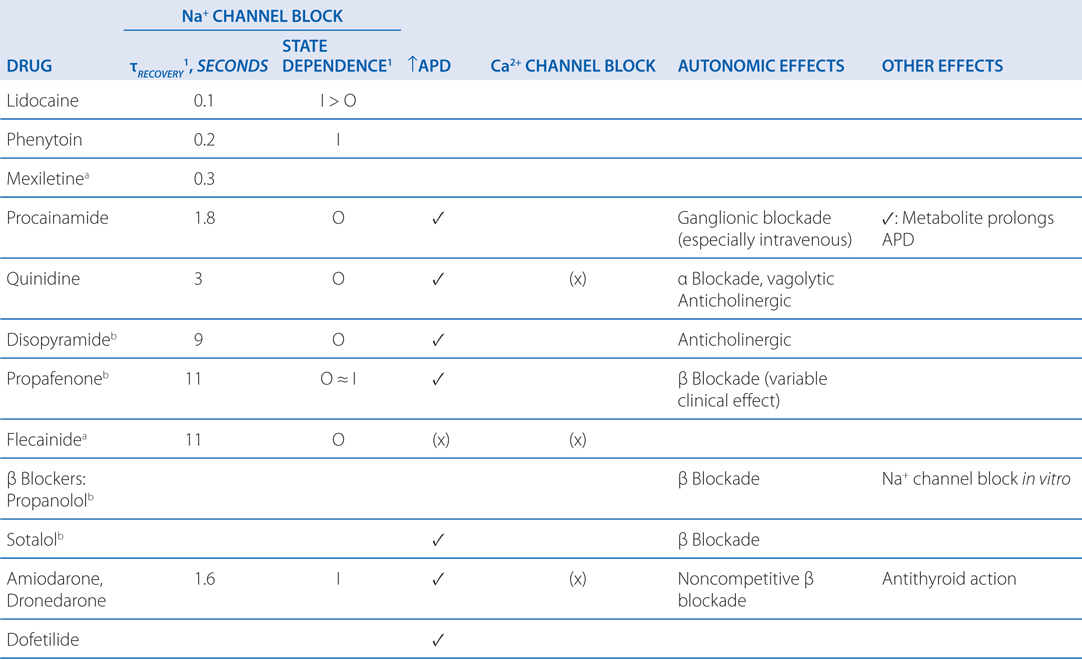
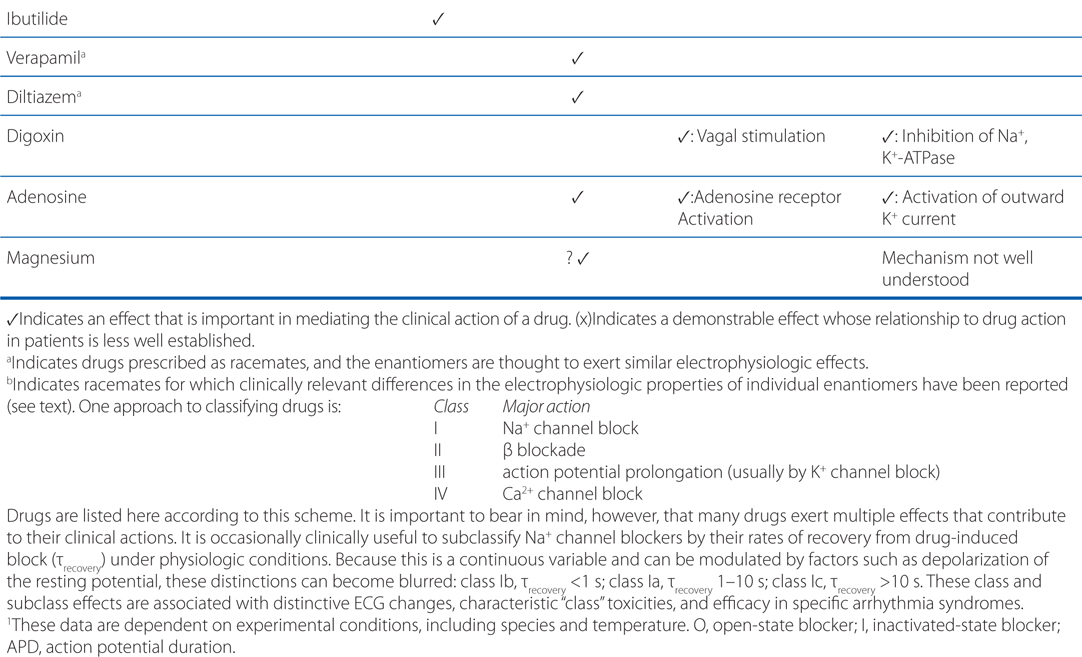
c. What treatments might be used in the long-term to prevent another episode of ventricular fibrillation in this patient?
The treatment of choice for long-term prevention of ventricular fibrillation is an implantable cardioverter-defibrillator (ICD; see Table 18-1). Such a device can be programmed to detect ventricular tachyarrhythmias and deliver a shock and/or pacing to maintain normal sinus rhythm. As an alternative or in addition to an ICD, drug therapy that includes a Na+ and/or K+ channel blocker could be used. Amiodarone is a drug of choice for chronic therapy because it has both Na+ and K+ channel blocking activities and has minimal negative inotropic effects.
A 38-year-old man complains of not having the energy to participate in strenuous sports that are part of his active lifestyle. He had been a competitive amateur cyclist but now has difficulty training. An ECG indicates he has atrial fibrillation.
a. What are the risks and benefits of starting this patient on antiarrhythmic drug therapy?
The risks of therapy include proarrhythmias as well as extracardiac side effects associated with long-term drug therapy. Pharmacological therapy that alleviates the patient’s atrial fibrillation may improve quality of life and reduce the risk of stroke, but may not reduce mortality.
b. What are the treatment options?
Because of the patient’s age and good health, nonpharmacological therapies should be considered including ablation and DC cardioversion (see Table 18-1). These have the potential to provide permanent or long-term termination of the arrhythmia. Antiarrhythmic drugs carry significant risks, and it might be safer to avoid any drug therapy, except for anticoagulant therapy, to reduce the risk of stroke.
A 12-year-old girl is brought to the ED with intermittent symptoms of dizziness and syncope. Her ECG shows that she has paroxysmal supraventricular tachycardia (PSVT).
a. What is the most likely arrhythmogenic mechanism causing the PSVT?
The most common mechanism causing PSVT is a reentrant circuit within or near the AV node (see Figures 29-7 and 29-8, in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition).
b. What is the drug of choice to rapidly terminate the PSVT?
Adenosine (IV) is the drug of choice to rapidly terminate PSVT because of its ability to inhibit conduction through the AV node (see Table 18-1). It acts through purinergic receptors to hyperpolarize AV nodal cells and has a very short half-life (<10 seconds). Other drugs that block AV nodal conduction include Ca2+ channel blockers (diltiazem and verapamil) and β adrenergic blockers, but adenosine is the drug of first choice because of its short half-life. Drugs that increase vagal tone (digitalis, edrophonium, phenylephrine) are less commonly used AV nodal blockers.
c. What can be done to prevent recurrence of PSVT in this patient?
Oral AV nodal blocking agents are the drugs of choice to prevent recurrence of PSVTs (see Table 18-1). Flecainide and propafenone are also useful drugs because of their ability to alter the electrophysiological properties of the fast-conducting tissue in the reentrant circuit. Selective ablation of tissue that is critical to the maintenance of the reentrant circuit (eg, an accessory pathway) is a treatment of choice because it can result in a permanent cure.
A 62-year-old man who had a myocardial infarction 3 years ago now complains of episodes of light-headedness. His cardiologist determines that his symptoms are the result of nonsustained ventricular tachycardia (VT), and that he has developed moderate systolic heart failure.
a. What is the likely cause of this patient’s ventricular tachycardia?
This patient’s VT is likely due to a reentrant circuit near the site of the healed infarction (see Table 18-1).
b. What are the treatment options for this patient?
The treatment of choice for this patient is an ICD that can sense when the patient has VT or ventricular fibrillation and can pace or cardiovert the heart into normal sinus rhythm (see Table 18-1). Alternatively or in addition to the ICD, this patient might receive an antiarrhythmic drug. Because this patient has heart failure, antiarrhythmic drugs that decrease left ventricular function (eg, disopyramide and flecainide) should be avoided. Drugs that would be appropriate are amiodarone, dronedarone, and K+ channel blockers such as dofetilide.
c. The patient’s cardiologist prescribes amiodarone. What are the toxicities that might be seen with long-term therapy?
As with most antiarrhythmic agents, there are risks of long-term therapy with amiodarone that must be considered and mitigated, if possible. Although amiodarone is effective in treating a wide range of arrhythmias and is one of the few antiarrhythmic agents that has been shown to reduce mortality in clinical trials, it is associated with a large number of extracardiac toxicities. Serious toxicities (some of which are life threatening) include pulmonary toxicity, hepatotoxicity, effects on thyroid function, neuromuscular symptoms, dermatological reactions, and effects on vision. The risk of these toxicities is increased because the mean elimination half-life of amiodarone is more than 50 days.
KEY CONCEPTS
 Antiarrhythmic drugs act by altering cardiac ion fluxes, directly or indirectly.
Antiarrhythmic drugs act by altering cardiac ion fluxes, directly or indirectly.
 Most antiarrhythmic drugs have multiple mechanisms of action.
Most antiarrhythmic drugs have multiple mechanisms of action.
 Antiarrhythmic drugs can cause arrhythmias and can have other life-threatening side effects.
Antiarrhythmic drugs can cause arrhythmias and can have other life-threatening side effects.
 In choosing an antiarrhythmic drug therapy, the risks of therapy, as well as possible benefits, should be considered; patients who are asymptomatic may derive little benefit from taking a drug that carries significant risks.
In choosing an antiarrhythmic drug therapy, the risks of therapy, as well as possible benefits, should be considered; patients who are asymptomatic may derive little benefit from taking a drug that carries significant risks.
PRINCIPLES IN THE CLINICAL USE OF ANTIARRHYTHMIC DRUGS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree