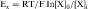Chapter 22 Antiarrhythmic Drugs
| Abbreviations | |
|---|---|
| ATP | Adenosine triphosphate |
| AV | Atrioventricular |
| CNS | Central nervous system |
| GI | Gastrointestinal |
| IV | Intravenous |
| NAPA | N-acetylprocainamide |
| NE | Norepinephrine |
| SA | Sinoatrial |
Therapeutic Overview
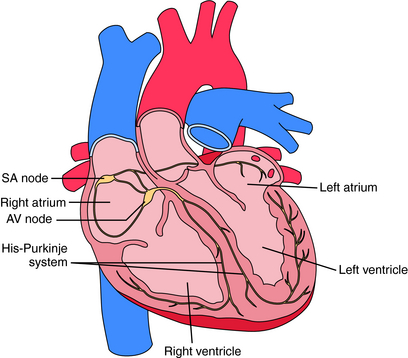
Electrical activation originates in specialized pacemaker cells of the sinoatrial (SA) node, located in the high right atrium near the junction with the superior vena cava (Fig. 22-1). After exiting the SA node, the electrical signal spreads rapidly throughout the atrium, leading to contraction. However, the atria are electrically isolated from the ventricles by the fibrous atrioventricular (AV) ring, with electrical propagation between atrium and ventricles occurring solely through the AV node and His-Purkinje system. The AV node delays the electrical impulse as it passes from atrium to ventricles, providing additional filling time before ejection. The signal then rapidly spreads throughout the ventricles using the Purkinje system, allowing a synchronized contraction.
Currently available antiarrhythmic drugs work by one of two mechanisms. They either directly alter
the function of ion channels that participate in a normal heartbeat, or they interfere with neuronal control. Although antiarrhythmic drugs are intended to restore normal sinus rhythm, suppress initiation of abnormal rhythms, or both, their use is hampered by the omnipresent risk of proarrhythmias. In the most famous example, the Cardiac Arrhythmia Suppression Trial showed that, even though ventricular arrhythmias predictive of sudden death could be suppressed by Na+ channel-blocking
| Therapeutic Overview | |
|---|---|
| Goal: To treat abnormal cardial impulse formation or conduction | |
| Effects: Modify ion fluxes, block Na+, K+, or Ca++ channels modify β adrenergic receptor-activated processes | |
| Drug Action | Uses |
| Na+ channel blockade | Paroxysmal supraventricular tachycardia, atrial fibrillation or flutter, ventricular tachycardia; digoxin-induced arrhythmias |
| β Adrenergic receptor blockade | Paroxysmal supraventricular tachycardia, atrial or ventricular premature beats, atrial fibrillation or flutter |
| Prolong action potentials and repolarization | Ventricular tachycardia, atrial fibrillation or flutter* |
| Ca++ channel blockade | Paroxysmal supraventricular tachycardia, atrial fibrillation or flutter |
| Other | |
| Adenosine | Paroxysmal supraventricular tachycardia |
| Digitalis glycosides | Atrial fibrillation or flutter with increased ventricular rate |
drugs, their use was associated with an increased incidence of sudden death.
Mechanisms of Action
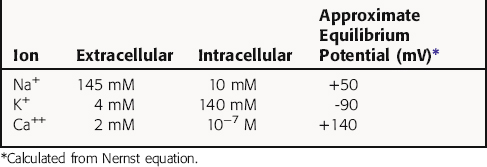
Cardiac myocytes, like other excitable cells, maintain a transmembrane electrical gradient, with the interior of the cell negative with respect to the exterior. This transmembrane potential is generated by an unequal distribution of charged ions between intracellular and extracellular compartments (Table 22-1). Ions can traverse the sarcolemmal membrane only through selective channels or via pumps and exchangers. The resting potential is an active, energy-dependent process, relying on these channels, pumps and exchangers, and large intracellular immobile anionic proteins. Critical components include the Na+/K+-adenosine triphosphatase (ATPase) and the inwardly rectifying K+ channel (IK). The Na+/K+-ATPase exchanges 3 Na+ ions from inside of the cell for 2 K+ ions outside, resulting in a net outward flow of positive charge.
where R = the gas constant; T = absolute temperature; F = the Faraday constant; and X is the ion in question. Because the usual intracellular and extracellular concentrations of K+ are 140 and 4 mM, respectively, its equilibrium potential is -94 mV. At rest, the sarcolemmal membrane is nearly impermeable to Na+ and Ca++ but highly permeable to K+. Therefore the resting potential of most cardiac myocytes approaches the equilibrium potential for K+ (-80 to -90 mV). However, the sarcolemmal membrane is dynamic, with a constantly changing permeability to various ions and resultant changes in membrane potential. The membrane potential at any given moment can be calculated based on knowledge of ion concentrations and permeabilities.
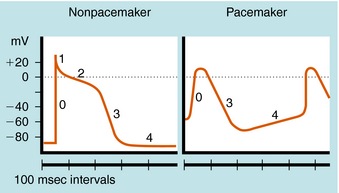
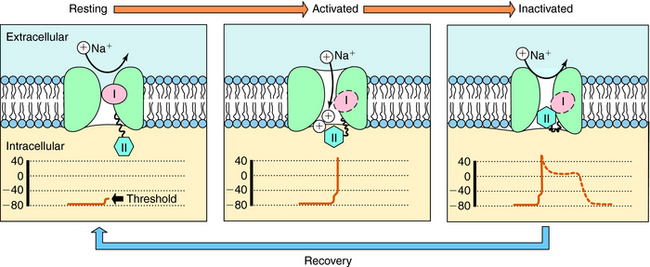
The cardiac action potential is divided into five phases as illustrated in Figure 22-2. The injection of current into a cardiac myocyte, or local current flow from an adjoining cell, can cause the membrane potential to depolarize (become less negative). If the resting potential exceeds a certain threshold, voltage-gated Na+ channels open (Fig. 22-3). Electrical and chemical gradients drive Na+ into the cell, making the membrane potential less negative. During phase 0, there is rapid depolarization of the action potential, Na+ influx is the dominant conductance, and the membrane potential approaches the equilibrium potential for Na+ (+64 mV). However, Na+ channels are open for only a very short time and close quickly. They also cycle through an inactivated state in which they are unable to open and participate in another action potential. Therefore, if a significant percentage of Na+ channels are in the inactivated state, the cell is refractory to further stimulation. The maximal rate of depolarization defines how fast electrical impulses can be passed from cell to cell, determining conduction velocity within a tissue. Slowing of conduction caused by inhibition of Na+ channels is the basis for the actions of Class I antiarrhythmic drugs. Action potentials in nonpacemaker cells are referred to as fast responses because their rate of depolarization is extremely rapid.
The voltage and time dependence of currents through individual ion channels are unique. Na+ channels open at more negative voltages than Ca++ channels, and current kinetics are quite different. Physical structures, known as activation and inactivation gates, help regulate the flow of ions. Because of these gates, Na+ channels are believed to exist in at least three distinct states during the cardiac action potential, as shown in Figure 22-3. At the resting potential, most Na+ channels are in a resting state, available for activation. Upon depolarization, most channels become activated, allowing Na+ to flow into the cell and cause a rapid depolarization. Na+ channels quickly become inactivated, limiting the time for Na+ entry to a few milliseconds or less.
Phase 2, or the plateau phase of the cardiac action potential, is one of its most distinguishing features. In contrast to action potentials in nerves and other cells (see Chapter 13), the cardiac action potential has a relatively long duration of 200 to 500 msec, depending on the cell (see Fig. 22-2). The plateau results from a voltage-dependent decrease in K+ conductance (the inward rectifier) and is maintained by the influx of Ca++ through Ca++ channels that inactivate only slowly at positive membrane potentials. During this phase, another outward K+ current, the delayed rectifier, is slowly activated, which nearly balances the maintained influx of Ca++. As a result, there is only a small change in potential during the plateau, because net current flow is small.
In a nonpacemaker cell, phase 4, or the resting potential, is characterized by a return of the membrane to its resting potential. Atrial and ventricular myocytes maintain a constant resting potential awaiting the next depolarizing stimulus, established by a voltage-activated K+ channel, IK1. The resting potential remains slightly depolarized relative to the equilibrium potential of K+, due to an inward depolarizing leak current likely carried by Na+. During the terminal portions of phase 3, and all of phase 4, voltage-gated Na+ channels are transitioning from the inactivated to the resting state and preparing to participate in another action potential. In a pacemaker cell, however, there is a slow depolarization during diastole. This brings the membrane potential near threshold for activation of a regenerative inward current, which initiates a new action potential (see Fig. 22-2). This is called phase 4 depolarization. In a pacemaker cell in the SA node, phase 4 depolarization brings the membrane potential to a level near the threshold for activation of the inward Ca++ current.
Mechanisms Underlying Cardiac Arrhythmias
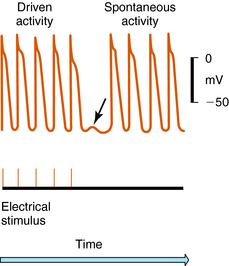
Arrhythmias result from disorders of impulse formation, conduction, or both. Several factors may contribute, such as ischemia with resulting pH and electrolyte abnormalities, excessive myocardial fiber stretch, excessive discharge of or sensitivity to autonomic transmitters, and exposure to chemicals or toxic substances. Disorders of impulse formation can involve either a change in the pacemaker site (e.g., sinus bradycardia or tachycardia) or the development of an ectopic pacemaker. Ectopic activity may arise as a consequence of the emergence of a latent pacemaker, because many cells of the conduction system are capable of rhythmic spontaneous activity. Normally these latent pacemakers are prevented from spontaneously discharging because of the dominance of the rapidly firing SA nodal pacemaker cells. Under some conditions, however, they may become dominant because of abnormal slowing of SA firing rate or abnormal acceleration of latent pacemaker firing rate. Such ectopic activity may result from injury due to ischemia or hypoxia, causing depolarization. Two areas of cells with different membrane potentials may result in current flow between adjacent regions (injury current), which can depolarize normally quiescent tissue to a point where ectopic activity is initiated. Finally, development of oscillatory afterdepolarizations can initiate spontaneous activity in normally quiescent tissue. These afterdepolarizations can occur at the end of phase 3 (Fig. 22-4) and, if large enough in amplitude, reach threshold and initiate a burst of spontaneous activity. Toxic concentrations of digitalis or norepinephrine (NE) can initiate such effects. This mechanism has also been proposed to explain ventricular arrhythmias in patients with the long QT syndrome.
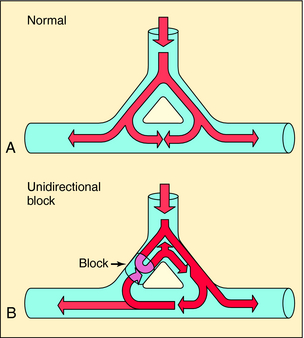
Disorders of impulse conduction can result in either bradycardia, as occurs with AV block, or in tachycardia, as when a reentrant circuit develops. Figure 22-5 shows an example of a hypothetical reentrant circuit. For a reentrant circuit to develop, a region of unidirectional block must exist, and the conduction time around the alternative pathway must exceed the refractory period of the tissue adjacent to the block. Before development of unidirectional block (see Fig. 22-5, A), impulse propagation initially branches as a result of the anatomical properties of the circuit. Some of these impulses collide and extinguish on the other side of the branch point. If an area of unidirectional block develops, impulses around the branch do not collide and become extinguished but may reexcite tissue proximal to the site of block, establishing a circular pathway for continuous reentry (see Fig. 22-5, B). Clinical examples include AV reentrant tachycardia (Wolff-Parkinson-White syndrome), AV nodal tachycardia, atrial flutter, and incisional/scar (atrial or ventricular) tachycardia. A long reentry pathway, slow conduction, and a short effective refractory period all favor reentrant circuits.
Antiarrhythmic drugs affect normal cardiac function and therefore have the potential for many serious adverse effects. In the most dramatic example, antiarrhythmic drugs have the potential to actually be proarrhythmic. Therefore treatment of a tachycardia, which is a nuisance clinically but not life-threatening, may initiate a life-threatening ventricular arrhythmia—truly a case of the cure being worse than the disease. Such potentially serious side effects require vigilance to ensure proper dosing, proper serum levels, a thorough knowledge of drug-drug interactions, and close follow-up.
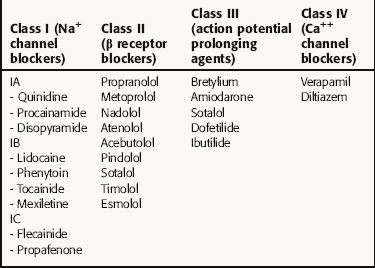
There is no universally accepted classification scheme for antiarrhythmic agents. The most commonly used scheme, the Vaughan-Williams classification, is based on the presumed primary mechanism of action of individual drugs (Table 22-2). This scheme classifies agents that block voltage-gated Na+ channels in class I, those with sympathetic blocking actions in class II, those that prolong action potential duration and refractoriness in class III, and those with Ca++ channel-blocking properties in class IV. However, classification is complicated by the fact that many drugs have multiple actions. As shown in Table 22-3, these drugs often have multiple effects on various targets. Although this scheme is useful in learning the properties of antiarrhythmic agents, all classifications are of limited use for treatment of arrhythmias because of their complex pathophysiology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree