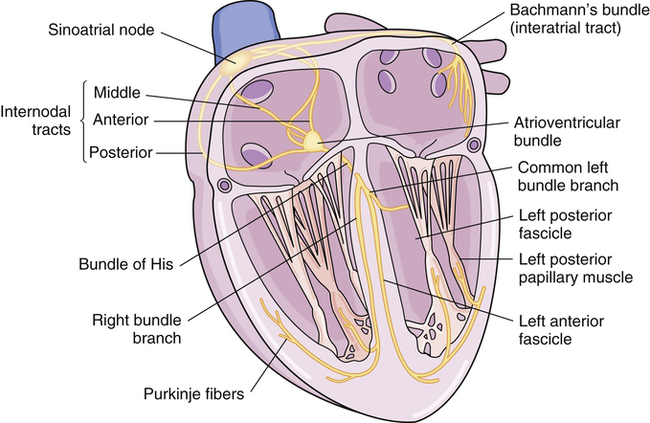
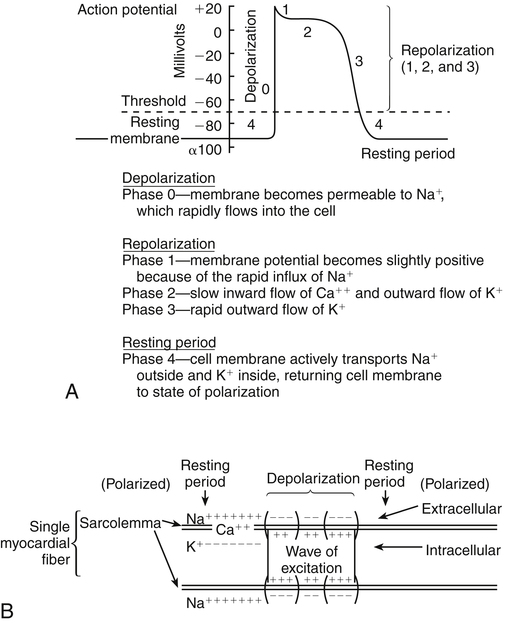
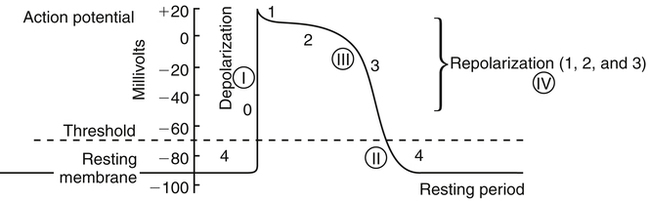
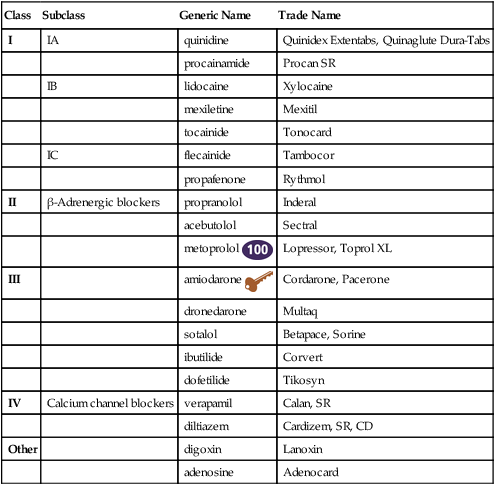
Chapter 23 The heart is composed of specialized myocardial muscle cells that have the capacity to generate an electrical potential (automaticity) and spread the electrical current from cell to cell (conductivity) (Figure 23-1). Many cells within the myocardium can serve as a pacemaker of the heart, but the primary pacemaker is found in the sinoatrial node. Electrical waves of depolarization spread through the atrium, the atrio-ventricular junction, and the bundle of His, and down the left- and right-bundle branches, producing synchronized atrial and ventricular muscular contractions. When the dominant pacemaker slows or does not fire, other cells take over and continue the heartbeat, although at a slower rate. Sometimes aberrant cells take over the pacemaker role, creating irregular rhythms and/or tachyarrhythmias. Action potential (Figure 23-2) refers to the difference in electrical charge across the myocardial cell membrane that results in polarization and depolarization. Depolarization is the electrical impulse that precedes the mechanical contraction of cardiac tissue. Repolarization is the recovery stage after muscle contraction. The mechanism of an arrhythmia is theoretically important in determining which drug will be effective. The two basic tachyarrhythmic mechanisms within the heart are (1) increased automaticity, resulting in an ectopic focus, and (2) reentry through abnormal conduction pathways. However, it is often clinically impossible to determine the mechanism without an electrophysiology mapping study. Arrhythmias that are caused by irritability or increased automaticity are treated with drugs that prolong the action potential, thus decreasing the rate at which impulses can be generated (Figure 23-3). Sustained ventricular tachycardia usually occurs as reentry, and it is treated with a drug that prolongs the effective refractory period. Antiarrhythmic drugs act to reduce electrical irregularity of the heart. They do this by altering the action potential of cardiac cells (Figure 23-4, Table 23-2). All antiarrhythmics have the potential to cause arrhythmia. In 1995, CAST (Cardiac Arrhythmia Suppression Trial) revealed the dangers of aggressive medical treatment of arrhythmias. TABLE 23-2 Mechanism of Action of Antiarrhythmic Medications
Antiarrhythmic Agents
Class
Subclass
Generic Name
Trade Name
I
IA
quinidine
Quinidex Extentabs, Quinaglute Dura-Tabs
procainamide
Procan SR
IB
lidocaine
Xylocaine
mexiletine
Mexitil
tocainide
Tonocard
IC
flecainide
Tambocor
propafenone
Rythmol
II
β-Adrenergic blockers
propranolol
Inderal
acebutolol
Sectral
metoprolol ![]()
Lopressor, Toprol XL
III
amiodarone ![]()
Cordarone, Pacerone
dronedarone
Multaq
sotalol
Betapace, Sorine
ibutilide
Corvert
dofetilide
Tikosyn
IV
Calcium channel blockers
verapamil
Calan, SR
diltiazem
Cardizem, SR, CD
Other
digoxin
Lanoxin
adenosine
Adenocard
Therapeutic Overview of Arrhythmias
Anatomy and Physiology
Pathophysiology
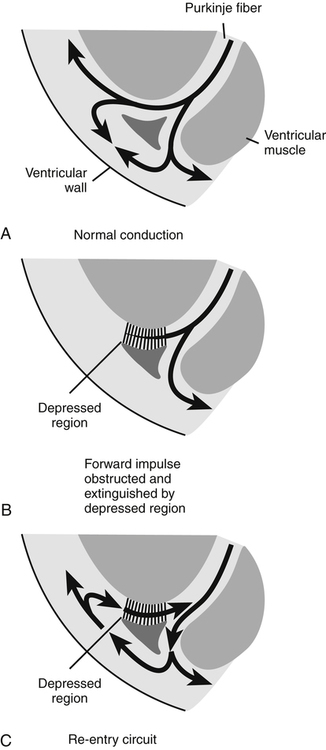
Mechanism of Action
Drug
Heart Rate
Automaticity of Foci
CONDUCTION VELOCITY
REFRACTORY PERIOD
Accessory Pathway
ECG CHANGES
AV Node
Ventricle
Atrium
Ventricle
PR Interval
QRS
QT Interval
IA
quinidine
±
↓
±
↓
↓↓
↑
↑
±
↑
↑
procainamide
±
↓
±
↓
↑
↑
↑↑
±
↑
↑
IB
lidocaine
0
↓
0
0
0
±
↑↓
0
0
↓
mexiletine
0
↓
0
0
0
↑
↑
0
0
0
tocainide
0
↓
0
0
↓
↓
↑
0
0
↓
IC
flecainide
0
↓
↓
↓↓
0
↑
↑↑
↑
↑↑
↑
propafenone
0
↓
↓
↓
0
↑
↑
↑
↑
↑
II (β-ADRENERGIC BLOCKERS)
propranolol
↓
↓
↓
↓
±
0
↑
↑
0
↓
metoprolol
↓
↓
↓
0
±
0
↑
↑
0
↓
III
amiodarone
↓
↓
↓
↓
↑
↑
↑
↑
↑
↑↑
dronedarone
IV (CALCIUM CHANNEL BLOCKERS)
verapamil
↓
↓
↓
0
0
0
0
↑
0
0
OTHER
digoxin
↓
↑
↓
↓
±
↓
↑↓
↑
0
↓
adenosine
↑
↓
↓
0
0
0
0
↑
0
0
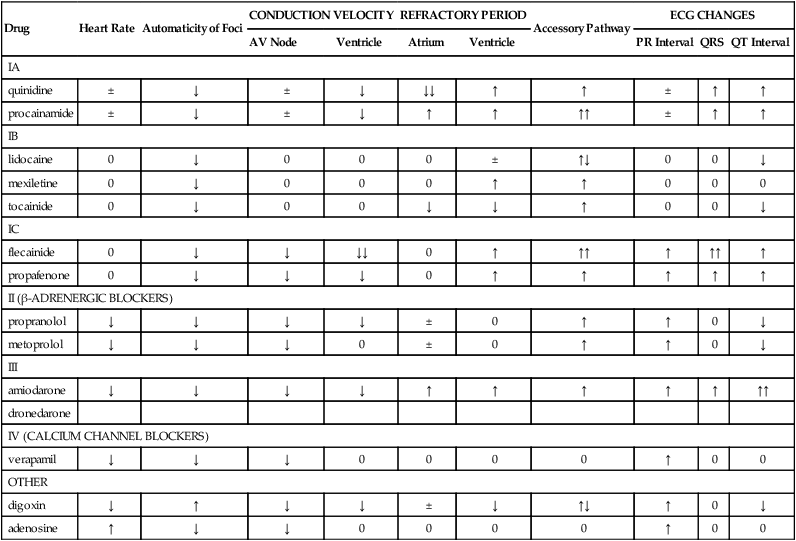
Antiarrhythmic Agents
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree