Angiomatoid Fibrous Histiocytoma
Khin Thway, BSc, MBBS, FRCPath
Key Facts
Terminology
Rare neoplasm of intermediate biologic potential
Most often arises in extremities of children and young adults
Histologically often confused with both benign and malignant lesions
3 characteristic translocations
2 identical to those of clear cell sarcoma
Clinical Issues
Slowly growing
Mostly indolent
15% recur
1% metastasize
Microscopic Pathology
Fibrous and lymphoplasmacytic cuff
Germinal centers
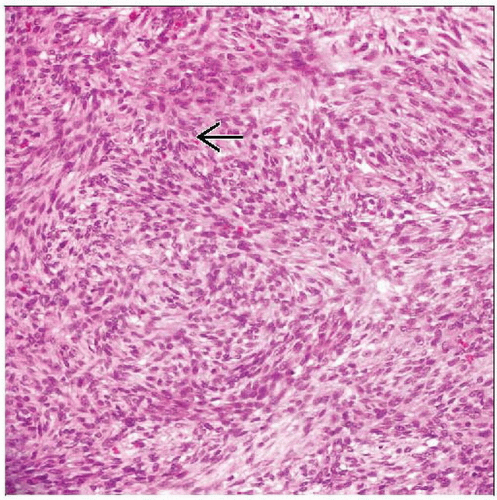
Sheets of histiocyte-like and spindle cells
Blood-filled spaces
Ancillary Tests
Desmin positivity in 1/2 of cases
Specific translocations
Top Differential Diagnoses
Pleomorphic sarcoma (malignant fibrous histiocytoma)
Aneurysmal benign fibrous histiocytoma
Palisaded (intranodal) myofibroblastoma
Kaposi sarcoma
Diagnostic Checklist
Age distribution
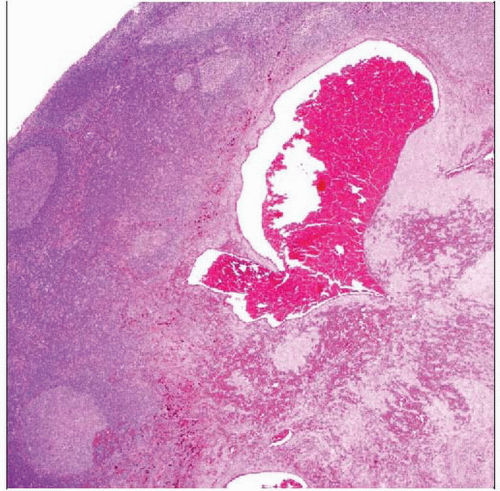 Hematoxylin & eosin shows a circumscribed lesion with a pronounced lymphoid cuff, including prominent germinal centers. This appearance may mimic that of a tumor metastatic to a lymph node. |
TERMINOLOGY
Abbreviations
Angiomatoid fibrous histiocytoma (AFH)
Synonyms
Originally angiomatoid “malignant” fibrous histiocytoma
Term “malignant” removed due to indolent behavior
Unrelated to malignant fibrous histiocytoma (MFH)/pleomorphic sarcoma group of neoplasms
Definitions
Rare neoplasm of intermediate biologic potential with 3 characteristic translocations
ETIOLOGY/PATHOGENESIS
Lineage Unknown
Endothelial or histiocytic differentiation not proven
Desmin expression suggests myoid or myofibroblastic differentiation
Postulated nodal fibroblastic reticulum cell differentiation
CLINICAL ISSUES
Epidemiology
Incidence
Rare
Accounts for approximately 0.3% of all soft tissue neoplasms
Age
Infancy to 8th decade
Predominantly in children and young adults
Gender
Slight female predilection
Site
Extremities
Trunk
Head and neck
1 primary intracerebral case reported
Usually superficial
Deep dermis and subcutis
Few arise deeply
Presentation
Slowly growing, painless mass
Usually small
Most often 2-4 cm
Constitutional symptoms in subset
e.g., malaise, pyrexia, and anemia
Possible tumoral cytokine production
Treatment
Surgical approaches
Wide excision
Usually curative
Radiotherapy and chemotherapy
For rare metastatic or unresectable tumors
Prognosis
Excellent in most cases
Majority of lesions indolent
Regional recurrence rate up to 15%
Metastasis rate of approximately 1%
Rare cause of death
No firm morphologic or clinical indicators of behavior
Infiltrative margin and deep location can predict recurrence
MACROSCOPIC FEATURES
General Features
Firm
Circumscribed
Blood-filled cystic cavities
Sections to Be Submitted
Lesion should be thoroughly sampled
Features, such as lymphoid cuff, may only be present focally
Small lesions should be submitted in entirety
MICROSCOPIC PATHOLOGY
Histologic Features
Circumscribed
Lobulated
Fibrous pseudocapsule
Dense peripheral lymphoplasmacytic cuff in up to 80%
Cellular tumor
Cells with bland, vesicular, ovoid to spindled nuclei
Sheets
Short fascicles
Occasional storiform patterns
Ovoid or spindle forms may predominate
Mitoses infrequent
Hemorrhagic cavities
No endothelial lining
Some show marked pleomorphism and mitotic activity
Giant cells in some cases
Predominant Pattern/Injury Type
Circumscribed
Cystic, macroscopic
Predominant Cell/Compartment Type
Mesenchymal
ANCILLARY TESTS
Immunohistochemistry
Desmin positivity
Approximately half of cases
Strong cytoplasmic expression
Scattered desmin(+) cells may be present within lymphoid proliferation
Tumors negative for skeletal muscle markers
e.g., myogenin and MYOD1
Epithelial membrane antigen (EMA)
Just under half of cases
CD68
Frequent but nonspecific
CD99
Frequent but nonspecific
Very occasional “intermediate” CD34 expression reported
Usually negative
Other vascular endothelial markers also usually absent
Cytogenetics
3 characteristic translocations identified
t(2:22)(q33:q12)
EWSR1-CREB1
Most common
t(12:16)(q13:p11)
FUS-ATF1
t(12:22)(q13:q12)
EWSR1-ATF1
Latter 2 translocations are identical to those of clear cell sarcoma (CCS)
CCS is morphologically and clinically distinct neoplasm
No correlation between type of fusion gene and clinicopathologic features
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree