INTRODUCTION
LEARNING OBJECTIVES
After studying this chapter you should be able to:
Establish a differential diagnosis of pancytopenia.
Classify the primary bone marrow disorders.
Explain the pathogenesis of aplastic anemia and the principles underlying its treatment.
It is clear from the information presented in Chapter 2 that the regulated production of circulating blood cells depends on the presence of an adequate number of functional multipotent hematopoietic stem cells. Accordingly, disease processes that either injure stem cells directly or compromise their environmental niche within the bone marrow result in a decrease in the numbers and sometimes the function of circulating blood cells. Thus, these bone marrow disorders often give rise to pancytopenia, a reduction in the numbers of circulating red cells, leukocytes, and platelets.
A variety of disorders can cause pancytopenia (Table 4-1). Because red cells, leukocytes, and platelets are all produced in the bone marrow, it is no surprise that microscopic examination of this organ by aspiration and biopsy is critical in evaluating patients with pancytopenia. As shown in the italicized portions of Table 4-1, this chapter will cover disorders involving injury to hematopoietic stem cells as well as those characterized by perturbation of the marrow microenvironment due to infiltration by cells that are extrinsic to the marrow (myelophthisis). Severe pancytopenia is a hallmark of marrow aplasia, whereas more variable degrees of cytopenia are encountered in myelophthisic disorders. Additional causes of pancytopenia listed in Table 4-1 are covered in other chapters of this book.
| Category | Disorder |
|---|---|
| Hematopoietic stem cell injury | Aplastic anemia* Idiopathic* Immune mediated* Secondary to drugs, toxins, irradiation, viruses* Fanconi anemia* |
| Clonal hematopoietic cell mutation | Acute leukemia (Chapter 21) Myelodysplasia (Chapter 20) Paroxysmal nocturnal hemoglobinuria (Chapter 11) |
| Myelophthisis (bone marrow infiltration) | Metastatic cancer* Granulomatous disorders, tuberculosis* Lymphoma (Chapter 22) Myelofibrosis (Chapter 20) |
| Defective maturation | Megaloblastic anemias (Chapter 6) |
| Enhanced peripheral destruction | Hypersplenism (Chapter 1) Autoimmune disorders, systemic lupus erythematosus, etc. Hemophagocytic lymphohistiocytosis (Chapter 18) |
APLASTIC ANEMIA
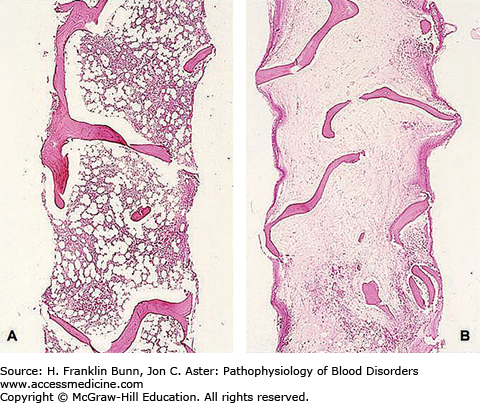
Because over 95% of the cells in the bone marrow are progeny of hematopoietic stem cells, injury to these cells will result in a marked decrease in cellularity. Figure 4-1 compares a low-magnification view of normal bone marrow with that of bone marrow from a patient with severe aplastic anemia. In this specimen (Fig. 4-1B) it would be difficult to identify any cells of the erythroid, myeloid, or megakaryocyte lineages. The few cells that can be identified are a mix of lymphocytes, plasma cells, and marrow stroma that includes endothelial cells and fibroblasts.
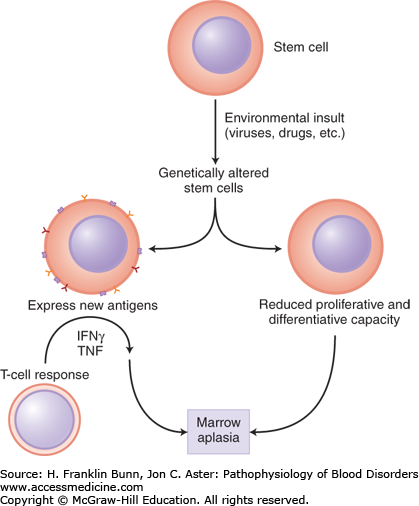
Marrow aplasia is thought to arise from genetic alterations in hematopoietic stem cells, either spontaneous mutations or mutations induced by an extrinsic insult such as a drug, toxin, or viral infection. As shown in Figure 4-2, the genetic damage may either directly impair the stem cell’s capacity for proliferation and differentiation or indirectly affect hematopoiesis by the induction of neoantigen expression in the stem cell and its progeny, which then triggers immune destruction via recruitment of cytotoxic T lymphocytes.
FIGURE 4-2
Pathogenesis of marrow aplasia. Damage to the genome of stem cells may either cause defects that impair proliferation and differentiation or induce formation of neoantigens that trigger immune destruction. (Red Blood Cell and Bleeding Disorders. In: Kumar V, Abbas, AK, Fausto N, Aster JC, eds. Robbins Pathologic Basis of Disease. Philadelphia, U.S.A.: Elsevier; 2010: p. 663.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree