Androgens
TESTOSTERONE AND OTHER ANDROGENS
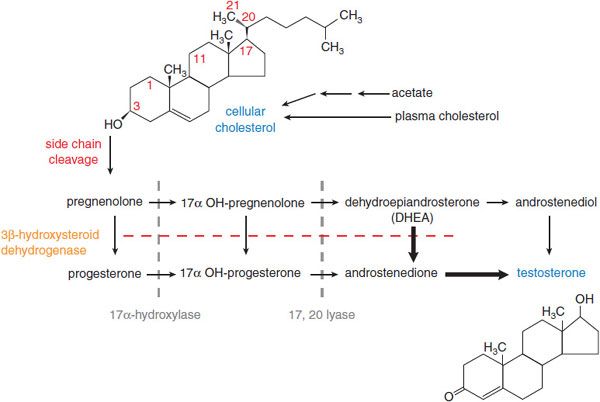
In men, testosterone is the principal secreted androgen. Leydig cells synthesize the majority of testosterone by the pathways shown in Figure 41–1. In women, testosterone also is the principal androgen and is synthesized in the corpus luteum and the adrenal cortex by similar pathways. The testosterone precursors androstenedione and dehydroepiandrosterone are weak androgens that can be converted peripherally to testosterone.
Figure 41–1 Pathway of synthesis of testosterone in the Leydig cells of the testes. In Leydig cells, the 11 and 21 hydroxylases (present in adrenal cortex) are absent but CYP17 (17 α-hydroxylase) is present. Thus, androgens and estrogens are synthesized; corticosterone and cortisol are not formed. Bold arrows indicate favored pathways.
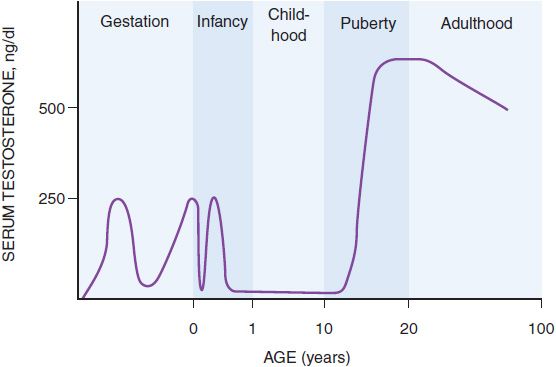
SECRETION AND TRANSPORT OF TESTOSTERONE. Testosterone secretion is greater in men than in women at almost all stages of life, a difference that explains many of the other differences between men and women. In the first trimester in utero, the fetal testes begin to secrete testosterone, the principal factor in male sexual differentiation, probably stimulated by human chorionic gonadotropin (hCG) from the placenta. By the beginning of the second trimester, the serum testosterone concentration is close to that of mid-puberty, ~250 ng/dL (Figure 41–2). Testosterone production then falls by the end of the second trimester, but by birth the value is again ~250 ng/dL, possibly due to stimulation of the fetal Leydig cells by luteinizing hormone (LH) from the fetal pituitary gland. The testosterone value falls again in the first few days after birth, but it rises and peaks again at ~250 ng/dL at 2-3 months after birth and falls to <50 ng/dL by 6 months, where it remains until puberty. During puberty, from ~12 to 17 years of age, the serum testosterone concentration in males increases so that by early adulthood the serum testosterone concentration is 500 ng/dL to 700 ng/dL in men, compared to 30 ng/dL to 50 ng/dL in women. The magnitude of the testosterone concentration in the male is responsible for the pubertal changes that further differentiate men from women. As men age, their serum testosterone concentrations gradually decrease, which may contribute to other effects of aging in men.
Figure 41–2 Schematic representation of the serum testosterone concentration from early gestation to old age.
LH, secreted by the pituitary gonadotropes (see Chapter 38), is the principal stimulus of testosterone secretion in men, perhaps potentiated by follicle-stimulating hormone (FSH), also secreted by gonadotropes. The secretion of LH by gonadotropes is positively regulated by hypothalamic gonadotropin-releasing hormone (GnRH); testosterone directly inhibits LH secretion in a negative feedback loop. LH is secreted in pulses, which occur approximately every 2 h and are greater in magnitude in the morning. The pulsatility appears to result from pulsatile secretion of GnRH from the hypothalamus. Testosterone secretion is likewise pulsatile and diurnal, the highest plasma concentrations occurring at ~8 A.M. and the lowest at ~8 P.M. The morning peaks diminish as men age. Sex hormone-binding globulin (SHBG) binds ~40% of circulating testosterone with high affinity, rendering the bound hormone unavailable for biological effects. Albumin binds almost 60% of circulating testosterone with low affinity, leaving ~2% unbound or free. In women, LH stimulates the corpus luteum (formed from the follicle after release of the ovum) to secrete testosterone. Under normal circumstances, however, estradiol and progesterone, not testosterone, are the principal inhibitors of LH secretion in women.
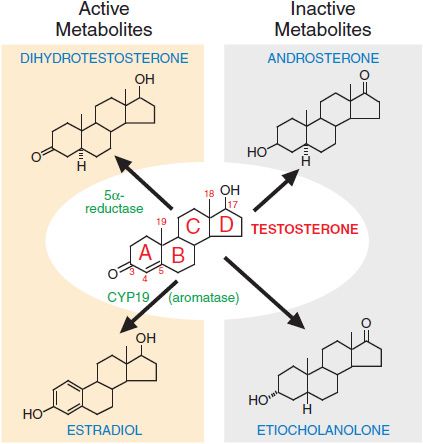
METABOLISM OF TESTOSTERONE TO ACTIVE AND INACTIVE COMPOUNDS. Testosterone has many different effects in tissues, both directly and through its metabolism to dihydrotestosterone and estradiol (Figure 41–3). The enzyme 5α-reductase catalyzes the conversion of testosterone to dihydrotestosterone. Dihydrotestosterone binds the androgen receptor (AR) with higher affinity than testosterone and activates gene expression more efficiently. Two forms of 5α-reductase have been identified: type I, which is found predominantly in nongenital skin, liver, and bone; and type II, which is found predominantly in urogenital tissue in men and genital skin in men and women. The enzyme complex aromatase, present in many tissues, catalyzes the conversion of testosterone to estradiol. This conversion accounts for ~85% of circulating estradiol in men; the remainder is secreted directly by the testes. Hepatic metabolism converts testosterone to the biologically inactive compounds androsterone and etiocholanolone (see Figure 41–3). Dihydrotestosterone is metabolized to androsterone, androstanedione, and androstanediol.
Figure 41–3 Metabolism of testosterone to its major active and inactive metabolites.
PHYSIOLOGICAL AND PHARMACOLOGICAL EFFECTS OF ANDROGENS
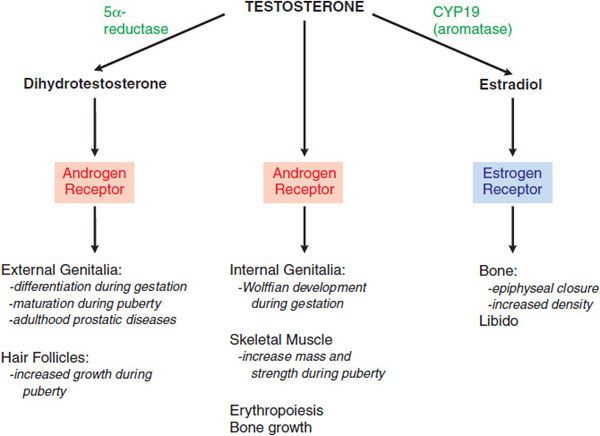
Testosterone is the principal circulating androgen in men. The varied effects of testosterone are due to its ability to act by at least 3 mechanisms: by binding to the AR; by conversion in certain tissues to dihydrotestosterone, which also binds to the AR; and by conversion to estradiol, which binds to the estrogen receptor (Figure 41–4).
Figure 41–4 Direct effects of testosterone and effects mediated indirectly via dihydrotestosterone or estradiol.
EFFECTS THAT OCCUR VIA THE ANDROGEN RECEPTOR. Testosterone and dihydrotestosterone act as androgens via a single AR, a member of the nuclear receptor superfamily and is designated as NR3A.
In the absence of a ligand, the AR is located in the cytoplasm associated with a heat-shock protein complex. When testosterone or dihydrotestosterone binds to the ligand-binding domain, the AR dissociates from the heat-shock protein complex, dimerizes, and translocates to the nucleus. The dimer then binds via the DNA-binding domains to androgen response elements on certain responsive genes. The ligand-receptor complex recruits coactivators and acts as a transcription factor complex, stimulating or repressing expression of those genes.
Mutations in the hormone or DNA-binding regions of AR result in resistance to the action of testosterone, beginning in utero. Male sexual differentiation therefore is incomplete, as is pubertal development. Other AR mutations occur in patients with spinal and bulbar muscular atrophy, known as Kennedy disease. These patients have an expansion of the CAG repeat, which codes for glutamine, at the amino terminus of the molecule. The result is very mild androgen resistance, manifest principally by gynecomastia, and progressively severe motor neuron atrophy. The mechanism by which the neuronal atrophy occurs is unknown. Other mutations in AR may explain why metastatic prostate cancer often regresses initially in response to androgen deprivation treatment, but then becomes unresponsive to continued deprivation. AR continues to be expressed in androgen-independent prostate cancer, and its signaling remains active. The ligand-independent signaling may result from mutations in the AR gene or changes in AR coregulatory proteins. In some patients resistant to standard androgen deprivation therapy, the tumor responds to further depletion of androgens by inhibitors of adrenal androgen synthesis, such as abiraterone.
EFFECTS THAT OCCUR VIA THE ESTROGEN RECEPTOR. Certain effects of testosterone are mediated by its conversion to estradiol, catalyzed by CYP19.
In the rare males deficient in CYP19 or the estrogen receptor, the epiphyses do not fuse and long-bone growth continues indefinitely; moreover, such patients are osteoporotic. Estradiol corrects the bone abnormalities in patients with aromatase deficiency but not in those with an estrogen-receptor defect. Because men have larger bones than women, and bone expresses the AR, testosterone also may have an effect on bone via the AR. Administration of estradiol to a male with CYP19 deficiency can increase libido, suggesting that the effect of testosterone on male libido may be mediated by conversion to estradiol.
EFFECTS OF ANDROGENS AT DIFFERENT STAGES OF LIFE
In Utero. When the fetal testes, stimulated by hCG, begin to secrete testosterone at about the eighth week of gestation, the high local concentration of testosterone around the testes stimulates the nearby Wolffian ducts to differentiate into the male internal genitalia. In the anlage of the external genitalia, testosterone is converted to dihydrotestosterone, which causes the development of the male external genitalia. The increase in testosterone at the end of gestation may result in further phallic growth.
Infancy. The consequences of the increase in testosterone secretion by the testes during the first few months of life are not yet known.
Puberty. Puberty in the male begins at a mean age of 12 years with an increase in the secretion of FSH and LH from the gonadotropes, stimulated by increased secretion of GnRH from the hypothalamus. The
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree