Fig. 12.1
Normal microscopic anatomy of the bladder (actin immunohistochemical stain). The urothelium rests on a lamina propria composed of connective tissue, vessels, and nerves. A few small wisps of smooth muscle are present within, near medium caliber vessels. The underlying muscularis propria is composed of thick, compact smooth muscle bundles
The bladder urothelium is of endodermal origin, derived from the cranial portion of the urogenital sinus in continuity with the allantois. The lamina propria, the muscularis propria, and the adventitia develop from the adjacent splanchnic mesenchyme. While the mesonephric ducts contribute initially to the formation of the mucosa of the trigone, this is subsequently entirely replaced by endodermal epithelium of the urogenital sinus. During embryologic development, the allantois regresses completely, forming the urachus, an epithelial-lined tube which extends from the umbilicus to the apex (dome) of the bladder [3]. Before or shortly after birth, the urachus involutes, becoming a fibrous cord which is called the median umbilical ligament (Fig. 12.2); the term urachal remnant should be limited to those instances when remnants of epithelium persist within the median umbilical ligament. The epithelial lining of the urachus is urothelium, similar to that of the urinary bladder and ureter, but it frequently undergoes metaplastic change, mostly glandular.
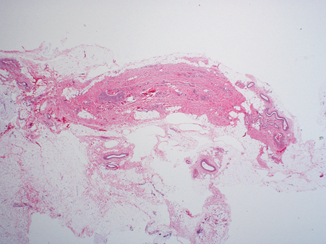

Fig. 12.2
Median umbilical ligament composed of dense fibro-connective tissue. At the time of prosecting, close examination of the bladder dome and anterior wall is required to visualize this remnant
In adults , the empty urinary bladder lies within the anteroinferior portion of the pelvis minor, inferior to the peritoneum, while in infants and children it is located partially within the abdomen, even when empty [4]. In adults, as the bladder fills it will distend and may extend into the abdomen. The bladder lies relatively free within the fibrofatty tissues of the pelvis except in the area of the bladder neck where it is firmly secured by the pubovesical ligaments in the female and the puboprostatic ligaments in the male [4, 5]. The relative freedom of the rest of the bladder allows for expansion superiorly as the organ fills with urine.
The empty bladder in an adult has the shape of a four-sided inverted pyramid and is enveloped by the vesical fascia [4]. The superior surface faces superiorly and is covered by the pelvic parietal peritoneum. The posterior surface, also known as the base of the bladder, faces posteriorly and inferiorly. It is separated from the rectum by the uterine cervix and the proximal portions of the vagina in females and by the seminal vesicles and the ampulla of the vasa deferentia in males. These posterior anatomic relationships are very important clinically. Since many bladder neoplasms arise in the posterior wall adjacent to the ureteral orifices , invasive tumor may extend into adjacent soft tissue and organs. The close anatomic relationship of organs explains why hysterectomy and partial anterior vaginectomy are commonly performed at the time of radial cystectomy in women. Similarly, perivesical soft tissue and seminal vesicle involvement is a bad prognostic marker in bladder carcinoma in males [6–8], a reflection of high pathologic stage. The latter is a rare occurrence but these patients do not appear to have a similarly bad prognosis unless prostatic stromal invasion is present. The two inferolateral surfaces of the bladder face laterally, inferiorly, and anteriorly and are in contact with the fascia of the levator ani muscles. The most anterosuperior point of the bladder is known as the apex or dome and it is located at the point of contact of the superior surfaces and the two inferolateral surfaces. It marks the point of insertion of the median umbilical ligament and consequently is the area where urachal carcinomas are located. Sometimes the insertion of the medial umbilical ligament is more towards the anterior apex.
The trigone is a complex anatomic structure located at the base of the bladder and extending to the posterior bladder neck. In the proximal and lateral aspects of the trigone, the ureters enter into the bladder (ureteral orifices) obliquely. The muscle underlying the mucosa in this region is a combination of smooth muscle of the longitudinal layer of the intramural ureter and detrusor muscle [9–13]. The intramural ureter is surrounded by a fibromuscular sheath (Waldeyer’s sheath), which is fused into the ureteral muscle (Fig. 12.3) . This fibromuscular tissue fans out in the area of the trigone and mixes with the detrusor muscle, thus fixing the intramural ureter to the bladder. As the bladder distends, the surrounding musculature exerts pressure on the obliquely oriented intramural ureter, producing closure of the ureteral lumen and thus avoiding reflux of urine. During micturition, there is contraction of the bladder musculature which also closes the intramural ureter. The bladder neck is the most distal portion of the bladder. It is the area where the posterior and the infero-lateral walls converge and open into the urethra (Fig. 12.4). The bladder neck is formed with contributions from the trigonal musculature (inner longitudinal ureteral muscle and Waldeyer’s sheath), the detrusor musculature, and the urethral musculature [9–14]. The internal sphincter is located in this general area, with major contributions from the middle circular layer of the detrusor muscle. In the male, the bladder neck merges with the prostate gland and one may occasionally observe several prostatic ducts present in this area (Fig. 12.4).
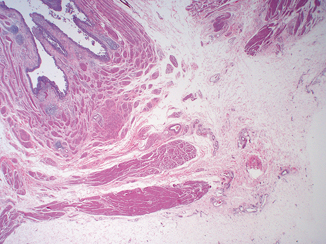
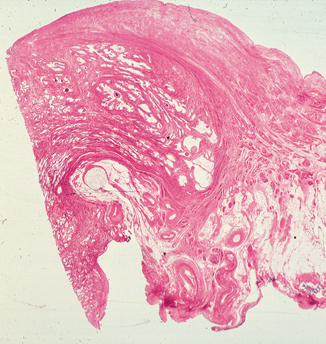

Fig. 12.3
Intramural ureter. At this site, the ureter exhibits a variable amount of lamina propria and longitudinal fibers of its own muscularis propria, which intermingle with the muscularis propria of the bladder

Fig. 12.4
Bladder neck. Notice displacement of muscle fibers of the muscularis propria towards the lumen of the bladder, compressing the lamina propria. There is intermingling of muscle fibers of the muscularis propria and the musculature of the prostate
The bladder bed (structures on which the bladder neck rests) is formed posteriorly by the rectum in males and vagina in females. Anteriorly and laterally it is formed by the internal obturator and levator ani muscles as well as the pubic bones. These structures may be involved in advanced tumors occupying the anterior, lateral, or bladder neck regions and render the patient inoperable except in a salvage setting, in order to relieve local symptoms.
Microscopic Anatomy
The urinary bladder , ureter, and renal pelvis for the most part have a similar anatomic composition, the innermost layer being an epithelial lining and, extending outwards, a lamina propria , smooth muscle (muscularis propria) , and adventitia (Fig. 12.1). The superior surface of the bladder comes in contact with parietal peritoneum and hence has a serosa. The anatomic landmarks are used clinically and pathologically to stage patients with urothelial cancer in order to choose therapy and estimate survival. For this reason, it is important to accurately identify them microscopically.
Urothelium
The urinary bladder is lined by urothelium, formerly referred to as “transitional mucosa.” The thickness of the urothelium will vary according to the degree of distension and anatomical location; in the contracted bladder, it is usually six to seven cells thick. One can identify three regions: the superficial or “umbrella” cells which are in contact with the urinary space, the intermediate cells, and the basal cells which lie on a basement membrane [15, 16] (Fig. 12.5). In practice, the thickness of the urothelium is dependent not only on the degree of distension but also on the plane on which the tissue is cut. If the cut is tangential to the basement membrane, it is possible to generate an artificially thick mucosa. For these and other reasons, we feel that urothelial thickness is at best of marginal utility in the evaluation of urothelial neoplasms. For the most part, the diagnosis of in situ urothelial carcinoma should be based of the degree of cytologic atypia and disorder.
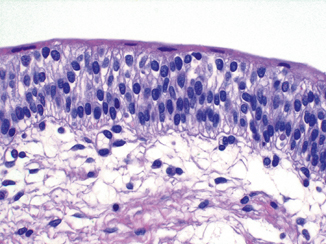

Fig. 12.5
Normal urothelium. The superficial (umbrella) cells span the luminal surface. The intermediate cell layer exhibits cells arranged perpendicular to the basement membrane, with regular nuclear contours, fine chromatin, and absent or minute nucleoli. In this section, the basal cell layer is inconspicuous but a very thin basement membrane is evident, as well as small capillaries
Superficial (umbrella) cells are large, elliptical cells which lie umbrella-like over the smaller intermediate cells [15–18]. They may be binucleated and have abundant eosinophilic cytoplasm. In a setting of chronic inflammation or intravesical therapy they can be quite atypical and hyperplastic, although the abundant eosinophilic cytoplasm remains. In the distended bladder, they become flattened and barely discernible. While the presence of these cells is taken as a sign of normalcy of the urothelium, one must be aware that they may become detached due to superficial erosion, during instrumentation or tissue processing in the prosecting area. It is possible to see umbrella cells overlying frank carcinoma so their presence or absence cannot be used as a determining factor of malignancy. Ultrastructural studies have shown the superficial urothelial cells to be quite unique, exhibiting what has been known as the “asymmetric unit membrane” (AUM) [17–21], which contains frequent invaginations. When the bladder distends, this unique feature allows for an increase in the surface area and maintenance of the structural integrity of the urothelium.
The intermediate cell layer may be up to five cells thick in the contracted bladder, where they are oriented with the long axis perpendicular to the basement membrane. The nuclei are oval and have finely stippled chromatin with absent or minute nucleoli. Longitudinal nuclear grooves are commonly present. These grooves are rarely, if ever, seen in urothelial carcinoma and certainly not in high-grade disease. Intermediate cells have ample cytoplasm which may be vacuolated. The cytoplasmic membranes are distinct and these cells are attached to each other by desmosomes. In the distended state or near an area of superficial erosion, this layer may be inconspicuous, only one cell thick and flattened. The basal layer is composed of cuboidal cells which are best seen in the contracted bladder. They lie on a thin but continuous basement membrane [22]. All normal urothelial cells may contain glycogen but only the superficial cells are occasional mucicarminophilic.
Urothelial Variants and Benign Urothelial Proliferations
While we have described the microscopic features of normal urothelium, we know there are many benign morphologic variants. Koss et al. studied 100 grossly normal bladders obtained at postmortem [23]. Of these, 93 % had either Brunn nests, cystitis cystica, or squamous metaplasia. It is important to understand this morphologic “plasticity” seen in benign urothelium since they can mimic variants of urothelial carcinoma (Table 12.1).
Table 12.1
Proliferative changes in the urothelium and the tumors they mimic
Benign variants | Carcinoma variants |
Brunn nests | Nested variant of urothelial carcinoma |
Cystitis cystica | Microcystic carcinoma |
Nephrogenic adenoma | Adenocarcinoma |
Cystitis glandularis with intestinal metaplasia | Enteric-type adenocarcinoma |
Inverted papilloma | Urothelial carcinoma with an inverted pattern of growth |
The most common benign urothelial variant is the formation of Brunn nests, which represent invaginations of the surface urothelium into the underlying lamina propria (Fig. 12.6a, b). In some cases, these solid nests of benign-appearing urothelium may lose continuity with the surface. If the proliferation of Brunn nests is profound, it can mimic a large nested variant of urothelial carcinoma. Brunn nests can become cystic due to accumulation of cellular debris or mucin and the term cystitis cystica has been coined to describe this phenomenon (Fig. 12.6a). The lining epithelium of these small cysts is composed of one or several layers of flattened urothelial or cuboidal epithelium. In can mimic microcystic urothelial carcinoma (Fig. 12.6b). In some cases, the epithelial lining undergoes glandular metaplasia, giving rise to what is called cystitis glandularis. The cells become cuboidal or columnar and mucin secreting; some are transformed into intestinal-type goblet cells (cystitis glandularis with intestinal metaplasia) (Fig. 12.7), and may be associated with extravasated mucin. Extreme examples can mimic mucinous adenocarcinoma. Brunn nests, cystitis cystica, and cystitis glandularis represent a continuum of proliferative or reactive changes and it is common to see all three in the same tissue sample. Most investigators believe that they occur as a result of local inflammatory insult [23–25]. Nevertheless, these proliferative changes are seen in the urothelium of patients with no evidence of local inflammation, so that it is possible that they also represent either normal histologic variants or the residual effects of old inflammatory processes [26, 27]. The high incidence of these proliferative changes in normal bladder suggests that they are not likely to be premalignant changes and that there is no cause-and-effect relationship between their presence and the appearance of bladder cancer. It is true that one or all of these changes are commonly present in biopsy specimens containing bladder cancer, but the coexistence may be coincidental or the cancer itself may be producing the local inflammatory insult that causes them. The fact that exceptional cases may occur in which carcinoma clearly arises within the epithelium of these reactive lesions does not alter this argument [28, 29].
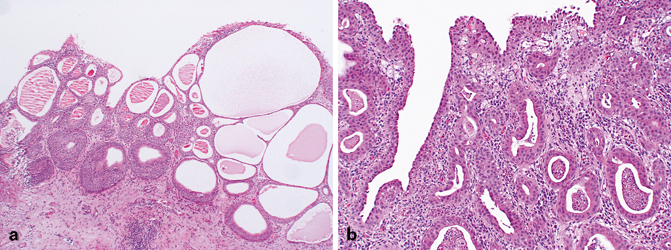
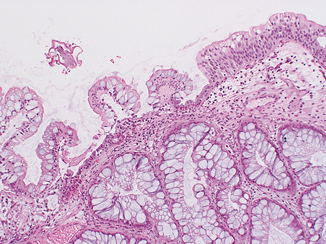

Fig. 12.6
a Florid proliferative cystitis characterized by Brunn nests and cystitis cystica. Notice the abrupt interface with the underlying lamina propria, suggesting circumscription. b Microcystic carcinoma. The nests of tumor are variable in size and shape and exhibit an infiltrative pattern

Fig. 12.7
Florid cystitis glandularis with intestinal metaplasia. Notice the transition from normal urothelium with prominent, sometimes vacuolated superficial umbrella cells to intestinal metaplasia of the surface urothelium containing cytologically banal goblet-type cells
Metaplasia refers to a change in morphology of one cell type into another which is considered aberrant for that location. Urothelium frequently undergoes either squamous or glandular metaplasia, presumably as a response to chronic inflammatory stimuli such as urinary tract infection, calculi, diverticula, or frequent catheterization [24, 27].
Squamous epithelium in the area of the trigone is a common finding in women. It is characterized by abundant intracytoplasmic glycogen and lack of keratinization, making it histologically similar to vaginal or cervical squamous epithelium. In this particular setting, most of us believe that squamous epithelium should be regarded as a variant of urothelium rather than metaplasia. Squamous metaplasia may occur at other sites and at times may undergo keratinization and even exhibit parakeratosis and a granular layer. Squamous metaplasia is not preneoplastic per se but patients with keratinizing squamous metaplasia must be monitored closely since some may progress to squamous carcinoma [30].
Cystitis glandularis is commonly encountered in a setting of chronic inflammation or irritation and also in cases of bladder exstrophy [31, 32]. The epithelium is composed of tall columnar cells with mucin-secreting goblet cells, strikingly similar to colonic or small intestinal epithelium in which one might identify even Paneth cells. As with squamous metaplasia, glandular metaplasia is not of itself a precancerous lesion but may eventually undergo neoplastic transformation in exceptional cases [32]. Patients should be monitored accordingly.
So-called nephrogenic adenoma is a distinct lesion characterized by aggregates of cuboidal or hobnail cells with clear or eosinophilic cytoplasm and small discrete nuclei without prominent nucleoli [33]. These cells line thin papillary fronds on the surface or form tubular structures within the lamina propria of the bladder. The tubules are often surrounded by a thickened and hyalinized basement membrane. Variable numbers of acute and chronic inflammatory cells are commonplace within the bladder wall.
The histogenesis of nephrogenic adenoma is still being debated. Some believe it develops secondary to an inflammatory insult or local injury [33–37]. It was originally described in the trigone and given its name because it was thought to arise from mesonephric rests. We now know that nephrogenic adenoma may occur anywhere in the urothelial tract, although it is most common in the bladder. It is important in that it may present as an exophytic mass mimicking carcinoma grossly and suggesting adenocarcinoma microscopically. The benign histologic appearance of the cells arranged in characteristic tubules surrounded by a prominent basement membrane should provide the correct diagnosis. A very interesting publication described nephrogenic adenomas of the bladder in patients that underwent renal transplantation [38]. The authors demonstrated the nephrogenic adenomatous lesions and the donor kidneys were clonal, suggestion that they developed through a process of shedding of donor renal tubule cells followed by implantation and proliferation within the bladder. Additional support for this hypothesis is postulated by other authors who have shown immunoreactivity for Pax-2, an antigen expressed in renal tubules [39, 40]. While this is interesting, it is unlikely to be the sole mechanism by which nephrogenic adenoma develops. The true specificity of Pax expression in this setting remains to be determined since it may very well be due to differentiation rather than histogenesis.
Inverted papillomas are relatively rare lesions that may occur anywhere along the urothelial tract and may be confused clinically and pathologically with urothelial carcinoma, specifically urothelial carcinoma with an inverted pattern of growth [41, 42]. In order of decreasing frequency, they occur in the bladder, renal pelvis, ureter, urethra, and renal pelvis [43–49]. Patients usually present with hematuria. Cystoscopically, the lesions are polypoid and either sessile or pedunculated. The mucosal surface is smooth or nodular without villous or papillary fronds. Microscopically, the surface transitional epithelium is compressed but otherwise unremarkable. It is undermined by invaginated cords and nests of transitional epithelium, which occupy the lamina propria . The accumulation of these endophytic growths gives the lesion its characteristic polypoid gross appearance. The urothelial cells forming the cords are benign, exhibiting normal maturation and few mitoses. They are similar to the cells of bladder papillomas , differing only in that the epithelial cords are endophytic and consequently more closely packed. Frequently, the cells are oval or spindle shaped. Epithelial nests may become centrally cystic, dilated, and even lined by cuboidal epithelium.
These cords of transitional epithelium in the lamina propria represent invaginations, not invasion. As such, there are no fibrous reactive changes within the stroma. Although mitotic figures can be seen, they are rare, regular, and located at or near the basal layer of the epithelium. Inverted papillomas are discrete lesions and do not exhibit an infiltrative border [43, 44]. One must be careful not to confuse a nested type of urothelial carcinoma infiltrating lamina propria with an inverted papilloma .
The etiology of inverted papilloma is unclear. Most investigators feel that, similar to other proliferative lesions such as Brunn nests and cystitis cystica, they are a reactive, proliferative process secondary to a noxious insult. They are not premalignant, although in exceptional cases they have been associated with carcinoma [45–47]. Given the rarity of this association, we consider it incidental.
Lamina Propria
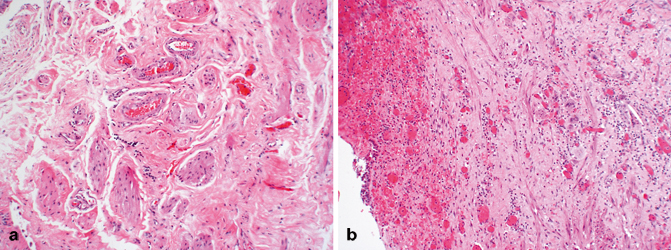
The lamina propria lies between the mucosal basement membrane and the muscularis propria (Fig. 12.1). It is composed of dense connective tissue containing a rich vascular network, lymphatic channels, sensory nerve endings, and a few elastic fibers [15, 19, 50]. In the deeper aspects of the lamina propria of the urinary bladder and ureter, the connective tissue is loose, allowing for the formation of thick mucosal folds when the viscus is contracted. Its thickness varies, not only with the degree of distention but also with the anatomic location. It is generally thinner in the areas of the trigone and bladder neck and thicker in the dome [51]. In fact, in patients with urinary outflow obstruction (i.e., prostatic hyperplasia) the bladder neck may contain muscularis propria directly beneath the mucosa with the lamina propria being virtually indiscernible (Fig. 12.4). Within the lamina propria lie intermediate-sized arteries and veins. Their exact location within the lamina propria may vary but they are usually roughly in the middle. Wisps and isolated fibers of smooth muscle are commonly found in the lamina propria, usually adjacent these vessels, and have been referred to as the muscularis mucosae (Fig. 12.8a) [52, 53]. These fascicles of smooth muscle are not connected to the muscularis propria and appear as isolated muscle fibers but may form a discontinuous thin layer of muscle. Uncommonly, these muscle fibers may present as a continuous layer of muscle within the lamina propria, thus forming a true muscularis mucosae [53]. Because the clinical management of tumors that involve the muscularis propria compared to those that invade only the lamina propria is so different, a pathologist must make every effort to make this distinction on biopsies and transurethral dissections (Fig. 12.8b). In cystectomy specimens, it is also important since it places the patient into a different prognostic category. Recent studies have described “hyperplastic” muscularis mucosae, defined as muscle bundles within the lamina propria that are more than four layers in thickness, thus mimicking muscularis propria (Fig. 12.9). These bundles may be arranged haphazardly in which individual fascicles are oriented in different directions or may have a compact arrangement with smooth outlines. When markedly thickened (hyperplastic), this pattern can easily be confused with muscularis propria (Fig. 12.10) [51, 54]. The anatomic relationship of these fibers to the overlying urothelium can be severely disrupted by inflammation, tumor-related desmoplasia, or prior therapeutic intervention, when they may be seen immediately beneath the basement membrane (Fig. 12.8b). Once again, every effort should be made to distinguish these muscle fibers of the muscularis mucosae from muscularis propria since a failure to do so will lead to errors in tumor staging and treatment. It goes without saying that a pathologist should not sign out a biopsy as “urothelial carcinoma invading muscle” because this phrase lacks critical information as to the depth of invasion, specifically what level of the bladder wall is involved by tumor .