Fig. 34.1
Descent of the testis. (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2014. All Rights Reserved.)
Embryology also explains the metastatic patterns of spread of testicular tumors. The metastatic tumors spread to the retroperitoneal (para-aortic, interaortocaval, paracaval) rather than inguinal lymph nodes as the vascular drainage follows descent from the genital ridge (Fig. 34.2). If there has been previous genital surgery, however, disruption to the drainage pattern may cause spread to the inguinal lymph nodes. Also, if there is failure of testicular descent as in intersex conditions, the lymph node drainage pattern of the testis may also be affected [5], although this is extremely rare .
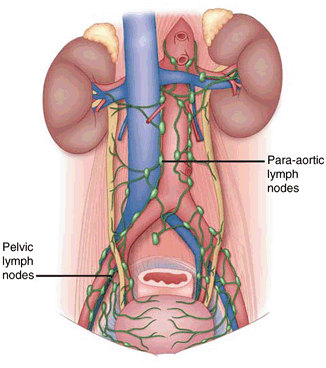

Fig. 34.2
Diagram showing pelvic and para-aortic lymph nodes
Testicular Dysgenesis Syndrome
It has been proposed by some that there is a strong causal association between some anatomical abnormalities and germ cell cancers. This has been termed the testicular dysgenesis syndrome (TDS) [6] and should not be confused with the disorder of sex development, gonadal dysgenesis, which is associated with streak morphology and ovarian differentiation. TDS remains a hypothesis, which is disputed by others [7], consisting of four defining conditions: impaired spermatogenesis , undescended testis, hypospadias, and testicular germ cell cancer. It suggests that an underlying genetic predisposition and environment, in utero factors, possibly endocrine, affect the developing fetus. This leads to abnormal Sertoli cell function and decreased Leydig cell function, with resulting germ cell maldevelopment and androgen deficiency, respectively, and to the four defining features (Fig. 34.3).
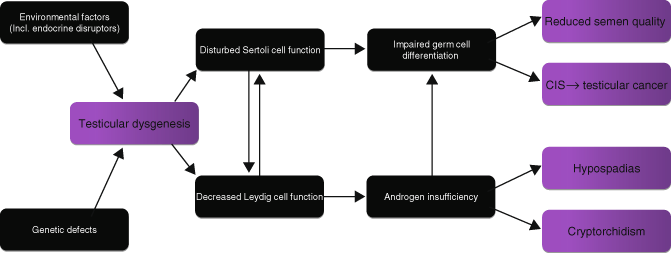

Fig. 34.3
The proposed pathogenesis of testicular dysgenesis syndrome
Anatomy of the Testis
The adult testis measures approximately 4.5 × 2.5 × 3 cm and weighs 20 g. Degrees of atrophy and testicular development throughout adolescence may be measured with an orchidometer, composed of a series of ovoids .
The testis is largely surrounded by an intrascrotal extension of peritoneal cavity (the processus vaginalis), which becomes the tunica vaginalis. Its visceral layer is apposed to the fibrous capsule of the testis, the tunica albuginea, and its parietal layer lines the most internal aspect of the scrotal wall. A small amount of fluid separates the visceral from the parietal layer. The embryology of testicular descent therefore results in mesothelium covering most of the testicular surface, with the visceral layer reflecting from the testis near the hilum and enveloping the testicular appendages. The testicular parenchyma is composed of seminiferous tubules with their components of various germ cells and Sertoli cells. The interstitium contains Leydig cells, blood and lymphatic vessels, and loose fibrous tissue. The seminiferous tubules connect into the rete testis at the hilum, and these anastomose with the efferent ductules that form the head of the epididymis, with the body and tail forming from convolutions of the Wolffian duct. The epididymis is attached to the posterior surface of the testis and gives rise to the ductus (vas) deferens (Fig. 34.4a–d). The testis is thus surrounded by easily identifiable histological structures: the testicular tunics, epididymis, and spermatic cord . Therefore, for radical orchidectomy cases, margins and tissues are easily identified and there is no need to use ink on macroscopic dissection. Staging is straightforward with appropriate tissue sample selection .
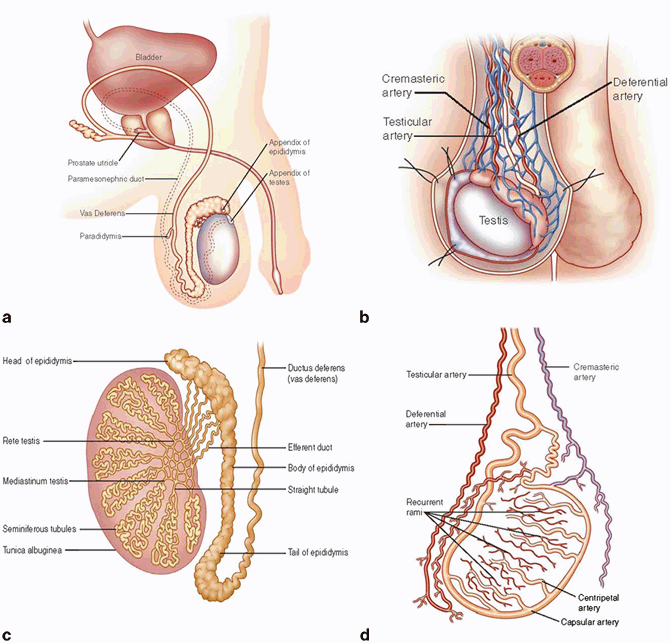

Fig. 34.4
a Cross section of the testis and the cord , demonstrating especially, the double-layered nature of the tunica vaginalis and the relationship of the lobules composed of seminiferous tubules to the rete and epididymis. b Demonstration of the testicular blood supply. The testicular artery arises directly from the abdominal aorta and descends through the inguinal canal, while the scrotum and the rest of the external genitalia are supplied by the internal pudendal artery. The testis has collateral blood supply from the cremasteric artery and the deferential artery. Lymphatic drainage of the testes follows the testicular arteries back to the para-aortic lymph nodes. c Cross section of the testis demonstrating the relationship of the lobules composed of seminiferous tubules to the rete testis, epididymis, and vas deferens. d A demonstration of the rich blood supply to the testis and rich anastomotic network of vessels
In recent years, there has been an increase in partial orchidectomy specimens [8]. This is for a number of reasons. Among them, the increased use of ultrasound has detected more testicular tumors of little clinical significance [9]. These include small sex cord-stromal tumors, Sertoli cell nodules, inflammatory conditions, non-neoplastic cysts, epidermoid cysts , and adenomatoid tumors . In the vast majority of these cases, a partial orchidectomy may be sufficient for cure, permitting better preservation of endocrine function and fertility and improved cosmesis. It is still a relatively infrequent operation, reserved particularly for men who may have already had contralateral orchidectomy or who have poor endocrine function or sperm counts. It is also technically limited to lesions that are distant from the rete and epididymis .
Partial orchidectomy specimens provide different challenges for the pathologist. They result in an excision specimen partially covered by mesothelium, which, as suggested above, is easily identified, but also having a testicular parenchyma as a surgical margin. On macroscopy, this must be inked so that it can be recognized as a surgical margin .
Microanatomy
Identification of the normal components of the seminiferous tubules is essential both to accurately identify abnormalities, of maturation, and to correctly recognize intratubular germ cell neoplasia. The seminiferous tubules contain germ cells in various stages of development from spermatogonia to spermatozoa along with the Sertoli cells (Fig. 34.5). Each tubule profile may show variable stages of maturation because development tends to occur in waves along the tubules. After the sperm are produced, they travel to the tubuli recti, which are found in the septa radiating out from the mediastinum of the testis (Fig. 34.6). The tubuli recti connect the seminiferous tubules with the rete testis.
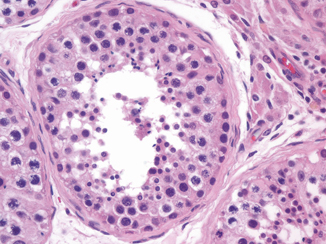
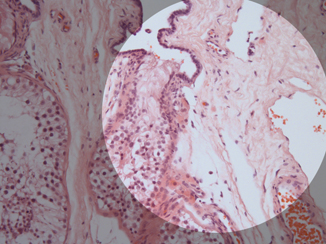

Fig. 34.5
A seminiferous tubule showing the different stages of spermatogenesis

Fig. 34.6
A seminiferous tubule connecting with the distal portion of the rete testis
Sertoli cells are distinguished by their location just above the basement membrane of the seminiferous tubule and the shape of the nucleus (Fig. 34.7). The Sertoli cell has an ovoid to triangular-shaped nucleus with a neatly punched-out red nucleolus. They form a ring around the basement membrane, between the spermatogonia and the other developing germ cells. The spermatogonia usually hug the basal lamina. They are large round cells with a pale staining round or ovoid nuclei. Dark and light forms, depending on subtle nuclear characteristics, have been noted [10], and the proportions of the different forms have been suggested to affect Leydig cell function.
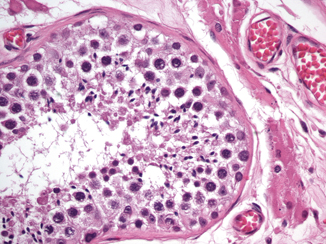

Fig. 34.7
Sertoli cell has an ovoid to triangular-shaped nucleus with a neatly punched-out red nucleolus. Spermatogonia are large round cells with a pale staining round or ovoid nuclei; they usually hug the basal lamina, and primary spermatocytes have a large floccular nucleus
Primary spermatocytes, which are undergoing the first meiotic division, have a large floccular nucleus, in which the individual condensed chromosomes can be sometimes seen forming elongated filamentous structures. Secondary spermatocytes are only rarely identified, as the second meiotic division occurs very rapidly. The early spermatid is small and round with a hyperchromatic nucleus and featureless chromatin. Later the spermatid becomes more conical. It then develops a flagellum and an even denser nucleus to become a spermatozoon (Fig. 34.7).
Relevance to Fertility Assessments
Two relatively new techniques allow the isolation and utilization of a single spermatozoon to fertilize a single egg for implantation in the uterus. These are performed in cases of non-obstructive oligo- or azoospermia and involve extraction of viable spermatozoa directly from the testis. While some practitioners attempt to aspirate spermatozoa with a technique similar to fine-needle aspiration, and then examine the extract for sperm, others use a biopsy technique, possibly more effective, called testicular sperm extraction (TESE). While some of the sample is used for fertilization, the parts of the biopsy not used may be sent for histopathological examination. The most important part of this examination, as far as the andrologists are concerned, is the identification of spermatozoa. When they are present in only small numbers, they can be better identified in histology sections than intraoperatively, and it suggests that a second TESE may be worthwhile if the initial fertility treatment failed. Therefore, the identification of the different meiotic cells in spermatogenesis is of great assistance [11]. Although some who work in this field attempted to “score the tubules” depending on the presence or absence of the developing elements of spermatogenesis (Table 34.1), the most relevant information required is merely the presence of spermatozoa and also the possible presence of intratubular germ cell neoplasia, unclassified (IGCNU).
Table 34.1
Johnson scoring of seminiferous tubules for fertility assessments
10—full spermatogenesis |
9—slightly impaired spermatogenesis, many late spermatids, disorganized epithelium |
8—less than five spermatozoa per tubule, few late spermatids |
7—no spermatozoa, no late spermatids, many early spermatids |
6—no spermatozoa, no late spermatids, few early spermatids |
5—no spermatozoa or spermatids, many spermatocytes |
4—no spermatozoa or spermatids, few spermatocytes |
3—spermatogonia only |
2—no germinal cells, Sertoli cells only |
1—no seminiferous epithelium |
IGCNU was initially termed “carcinoma in situ,” a nomenclature that is still used by some [12]. Nearly the entire array of malignant germ cell tumor elements has been identified in seminiferous tubules; however, the most common by far is intratubular germ cell neoplasia, unclassified. Identification of IGCNU is important because of its virtually uniform eventual progression to an invasive germ cell tumor. In testicular biopsies, it may be found as an incidental finding or at the same time as orchidectomy for germ cell neoplasia in those centers where the contralateral testis is biopsied [13]. However, as there are a number of mimics of germ cell neoplasia, especially classical seminoma, identification of IGCNU may greatly help facilitate a difficult diagnosis where there is a question of germ cell neoplasm versus another process.
Ectopic Tissue and Pseudomalignant Changes
Leydig cells may be present not just in the testicular parenchyma, between the seminiferous tubules, but also in the fibrous capsule of the tunica albuginea, rete testis, paratesticular soft tissue, and occasionally epididymis [14]. They are relatively inconspicuous unless there is a cause of Leydig cell hyperplasia, when they can become more prominent and possibly be mistaken for an invasive Leydig cell tumor . Leydig cells are frequently associated with nerves, which must not be misinterpreted as perineural invasion by a malignant tumor, especially if the testis harbors an otherwise innocuous Leydig cell tumor, which, however, would lack the usual features associated with malignant behavior in this neoplasm. Therefore, it is important to realize that Leydig cells may be present at “ectopic” locations .
Adrenocortical-like tissue can also be seen in ectopic foci, usually near the rete testis [15] and in the cord . Ectopic foci are generally entirely incidental; however, they become of great importance in the inherited adrenogenital syndrome or “congenital adrenal hyperplasia.” In this rare condition, there is gross hyperplasia of these adrenal cells. They often resemble Leydig cells, although they may have more voluminous cytoplasm, more prominent cytoplasmic pigment, lack Reinke crystals, and are associated with fibrosis [16]. While in the initial stages of congenital adrenal hyperplasia the lesions are usually confined to the rete testis, they may expand into the testicular parenchyma where they present as testicular masses. These “tumors” are extremely responsive to high-dose steroid suppression, and the first treatment is always medical, surgery being used only as a last resort due to pain. Unfortunately, cases of bilateral orchidectomy, rendering the patient castrate, for bilateral testicular “tumors” of the adrenogenital syndrome continue to occur; the pathologist needs to be aware of this condition and prevent unnecessary orchidectomy where possible.
Bizarre nuclear change in the epithelium of the epididymal tubules may cause concern for a malignant process. This phenomenon, however, has no clinical significance and appears to be of degenerative nature, entirely analogous to the much more common but similar finding in the seminal vesicles . The nuclei, although enlarged, have dense, smudgy chromatin and sometimes intranuclear cytoplasmic inclusions. Mitotic figures are absent. Along the same lines, complex cribriform arrangements of the glandular epithelium in the epididymal tubules may provoke concern for adenocarcinoma, but this finding is entirely within the spectrum of normal morphology of the epididymis.
Staging of Testis Cancer
The management of testicular tumors, particularly germ cell tumors, differs radically from many other of the genitourinary malignancies . Whereas accurate staging either on imaging or by histology is critical for prostatic adenocarcinomas or urothelial neoplasms, in the complex world of testicular tumors, the tumor type is of supreme importance, although staging of the primary lesion plays a secondary role for the pathologist. This is for a number of reasons. Firstly, the excellent prognosis of most germ cell tumors means that large randomized trials with staging being used to designate treatment are virtually impossible. Outcome data have to be available on thousands of patients to create enough treatment failures to yield significant differences in outcome based on pathological criteria. Secondly, the treating physicians are also likely to utilize non-pathological criteria, such as the levels of serum tumor markers and findings on retroperitoneal imaging, to assist with treatment choices, regardless of pathologic staging of the testicular primary .
The currently recommended staging system for testicular tumors is the AJCC-TNM classification [17], which is summarized in Table 34.2. It should be noted that because tumor markers play such a key role in the management of patients with germ cell tumors and have been shown to have prognostic importance, the degree of elevation of various serum markers is considered in the determination of the stage groups. In fact, any of the T stages can be clinical stage I–III, meaning that the serum markers or imaging not infrequently “trump” the pathological assessment. An even more clinically based system, the International Germ Cell Consensus (IGCC) classification, stratifies patients with non-seminomatous germ cell tumors (NSGCTs) into three risk groups (good, intermediate, and poor prognosis) on the basis of serum markers (measured prior to orchiectomy) and distribution of metastases (Table 34.3) .
Table 34.2




AJCC staging system for testicular cancer. Note that nodes imaging and serology also have a large role in determining the stage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


