Fig. 22.1
a This is a perifascial radical nephrectomy with massive main renal vein involvement by clear cell renal cell carcinoma . Gerota’s fascia is intact and visible as a thin delicate connective tissue envelope for the kidney and adrenal. It has been incised medially (arrow) exposing more clearly the hilar-perinephric fat. b This section shows Gerota’s fascia. It is a thin vascularized connective tissue layer that invests the peripheral perinephric fat located below. c The anterior pararenal space is thin, overlying the kidneys. Anterior to it is the peritoneum. In this radical nephrectomy, there is a portion of the peritoneum (between arrows) covering Gerota’s fascia
Peripheral Perinephric Fat Compartment
The peripheral perinephric fat contains the adrenal gland, and the hilar structures already mentioned. It surrounds the outer aspect of the kidney and is separated from the kidney by the fibrous renal capsule. The quantity of peripheral perinephric fat varies substantially in nephrectomy specimens, especially since adrenal-sparing procedures are often employed. Although it is common practice to weigh nephrectomy specimens, these data provide little useful information. For RCC to qualify as extending into this perinephric compartment, it should be in contact with adipocytes, or in loose connective tissue containing adipocytes.
Renal Parenchyma
The kidney consists of two basic components—the renal cortex and the renal medulla, also known as the renal pyramids because of their distinctive shape [3] (Fig. 22.2). The cortical tissue includes the columns of Bertin, portions of the cortex that dip deeply between the renal pyramids toward the renal sinus fat as mentioned above. The kidney is partially invested by the renal capsule, a dense fibrous tissue layer that covers the peripheral aspects of the kidney and extends a short distance into the renal hilum where it terminates (Figs. 22.2 and 22.3a). The cortical columns of Bertin that extend between the renal pyramids are in direct contact with the renal sinus without an intervening fibrous capsule (Fig. 22.3b). Assuming that the renal capsule provides some resistance to tumor extension outside the kidney into the peripheral perirenal fat, the absence of a capsule between the columns of Bertin and the sinus fat may represent a preferential site for extrarenal extension into the central perinephric sinus fat. This may be especially pertinent for tumors arising in the column of Bertin.
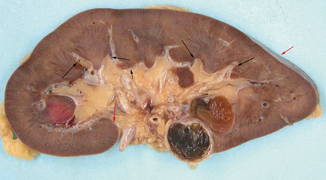
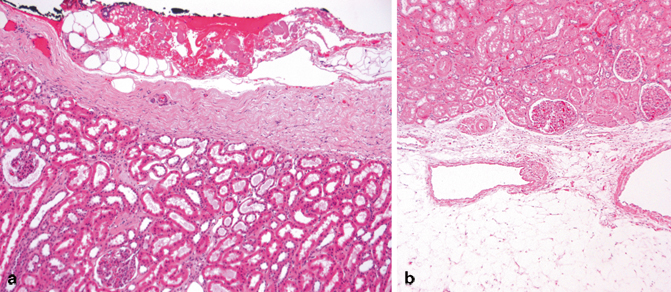

Fig. 22.2
This nephrectomy was bivalved through the lateral mid plane. Most of the perinephric fat was removed. A small portion of the renal capsule is visible to the upper right ( red arrow). The renal capsule curves into the renal sinus a short distance then terminates ( red arrow). The renal parenchyma consists of the renal cortex and medulla. The cortical columns of Bertin extend between the pyramids and are in direct contact with the renal sinus ( short arrow). The renal sinus is the central fatty compartment. Wedges of sinus fat extend toward the cortex between the papilla and the columns of Bertin ( long arrows)

Fig. 22.3
a This section shows the dense fibrous renal capsule. It represents a fibrous barrier between the peripheral perinephric fat and the peripheral renal cortex. b This image shows the interface between a column of Bertin and the renal sinus fat. Notice the absence of a fibrous capsule. The renal sinus fat with two veins is visible below. It is not uncommon for a delicate connective tissue layer to be present between the cortical tissue and the sinus fat as shown here. Involvement of this connective tissue layer is regarded as extension beyond the kidney (pT3a)
The renal cortex consists of two histological compartments—the cortical labyrinth and the medullary rays. The cortical labyrinth contains glomeruli, proximal and distal convoluted tubules, and the initial portion of the collecting ducts, as well as the renal arteries, veins, and lymphatics. The medullary rays contain parallel tubular segments that course down into the medulla and travel back up to the cortex. It is important to be familiar with the normal histology of the kidney because the nonneoplastic cortex provides a window into the presence of systemic diseases. Especially important are the common systemic diseases hypertension and diabetes—conditions, that when detected, may have greater prognostic implications than the neoplasm itself. The reader is referred to the recent reviews on this topic that have led to reporting recommendations regarding findings in the nonneoplastic cortex [4, 5].
Central Perinephric Sinus Fat Compartment
The renal sinus is the fatty compartment located within the central confines of the kidney (Fig. 22.2). Involvement of the renal sinus veins was recognized as the primary route of tumor dissemination in nephroblastoma in the early 1980s [6]. A similar role in tumor dissemination for RCC, however, was not shown until 2000 [7]. Inclusion of renal sinus involvement in RCC staging was first codified in the 2002 TNM formulation [8]. Extension into this perinephric compartment is now known to be the most common site of extrarenal extension by RCC. Therefore, understanding the anatomy and histology of this compartment is critical to the accurate staging of RCC [9, 10].
The renal sinus begins at the renal hilum and fills the space between the pelvicalyceal system and the renal parenchyma. The renal sinus has a complex three-dimensional structure [1, 2, 11]. There are pyramidal extensions of the sinus containing fat and interlobar vessels between the renal pyramids that separate the minor calyces from the columns of Bertin (Fig. 22.2). These slender cords of sinus approach within 1–1.5 cm of the renal capsule. Therefore, it is no surprise that sinus tissue is commonly present in partial nephrectomy specimens. This should be looked for grossly at the partial nephrectomy resection margin and when the specimen is sectioned (Fig. 22.4a, b) because sinus fat and sinus veins will occasionally be involved. Most renal pyramids and minor calyces angle toward the central portion of the renal sinus from the anterior and posterior planes of the kidney . Therefore, in a bivalved specimen and in kidney sections, the sinus tissue can be encountered completely surrounded by renal parenchyma (Fig. 22.5a, b).
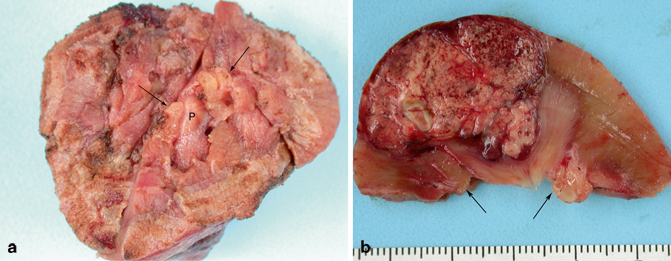
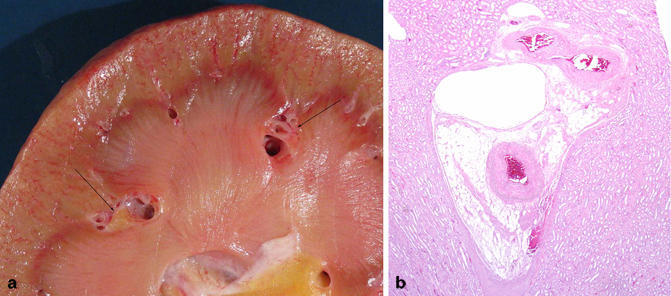

Fig. 22.4
a This image shows the surgical resection margin of a partial nephrectomy. Several renal papillae ( P) can be seen. In addition, two wedges of renal sinus fat are also visible ( arrows). b This cut surface of the above specimen shows a papillary RCC. Notice the two wedges ( arrows) of sinus fat flanking the renal pyramid

Fig. 22.5
a This bivalved kidney shows a compound renal pyramid in the center with two islands of renal sinus ( arrows) seen in cross section that are completely surrounded by renal parenchyma. b In this section of the kidney, there is an island of renal sinus surrounded by the cortex to the top and renal pyramids on both sides. There is a thin-walled interlobar vein adjacent to the cortex that would be easily accessible to a RCC if developing in this area
The renal cortex of the columns of Bertin is in direct contact with the renal sinus without an intervening capsule in contrast to the peripheral renal cortex as previously mentioned (Figs. 22.2 and 22.3b). The renal tubules of the cortical column of Bertin tissue may contact adipocytes directly, or may be separated by loose connective tissue fibers. Involvement of either constitutes the renal sinus involvement. The renal pyramids, by contrast, are nested within minor calyces and are not in direct contact with the renal sinus. When RCC involves the collecting system, it usually indicates sinus involvement because it would be uncommon, but not impossible, for a slender cord of tumor to breach only the papillary tip without also invading the renal sinus. The renal sinus’ defining attribute is its lush vascularity discussed in detail below.
Renal Parenchymal Vasculature
The renal parenchymal vasculature has two components [1–3, 11–14]. There is a minor system of small vessels, the stellate arteries and veins, which supply and drain, respectively, the superficial cortex through the renal capsule (Fig. 22.6a). These arteries and veins are themselves supplied by, and drain into, the major hilar vessels. An intravenous tumor in the peripheral perinephric fat represents a tumor that has gained access to the stellate system or may be there by retrograde extension from the hilar connection of the stellate veins (see below). It may be that the venous engorgement occasionally observed in tumor capsules represents this system of veins (Fig. 22.6b).
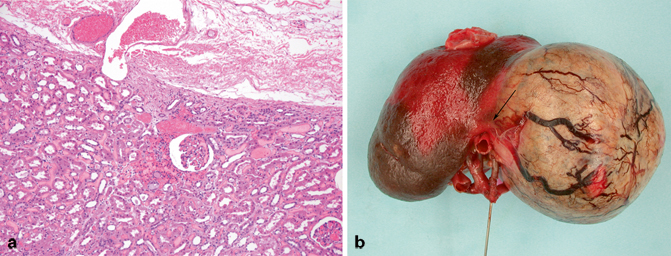

Fig. 22.6
a A small dilated stellate vein can be seen in this image traversing the fibrous renal capsule. b The perinephric fat and renal capsule have been removed from this nephrectomy specimen exposing the intact tumor capsule. Notice the engorged veins that drape across the tumor capsule. They disappear ( arrow) as they approach the hilum to drain into hilar veins
The major renal parenchymal vasculature resides in the central cortical labyrinth. The arteries and veins travel in parallel as they ascend from, and descend to, the renal sinus, respectively. The renal parenchymal veins are distinctive compared to veins in most other organs because they lack a smooth muscle media (Fig. 22.7a, b). They are essentially very large capillaries. The absence of a smooth muscle media assumes importance with respect to the recognition of retrograde cortical venous invasion and the possibility of multifocal tumors, issues addressed in Chap. 24.
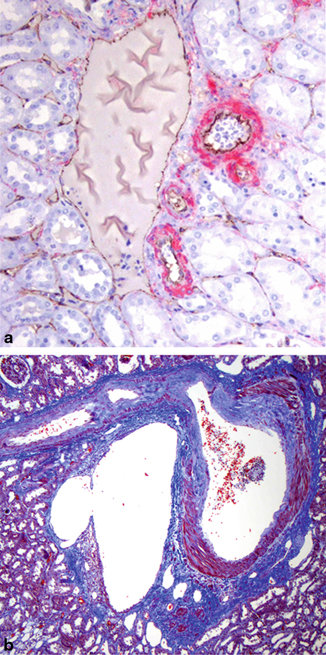

Fig. 22.7
a This image shows a cortical interlobular vein to the left with small interlobular arteries and arterioles to the right. The arteries and veins are always adjacent. Notice that the vein lacks a smooth muscle media and resembles the peritubular capillaries in the surrounding cortex. Red: actin smooth muscle; brown: CD 31 immunoperoxidase stains. b This is the corticomedullary junction. The cortex is to the top. Abundant smooth muscle can be seen in the media of the artery to the right. However, the arcuate vein to the left is devoid of medial smooth muscle. Masson’s trichrome stain
The interlobular veins of the cortex progressively enlarge as they approach the corticomedullary junction to form the arcuate veins. The arcuate veins drain into the interlobar veins that travel between the pyramid and enter the renal sinus. There are elaborate anastomoses between these large veins that encircle the renal pyramids and minor calyces. The interlobar veins converge within the renal sinus forming segmental veins that course anterior to the renal pelvis. Once veins enter the sinus, they acquire a smooth muscle media as discussed below. There are no arteries or veins in the renal medulla, only arterioles, venules, and capillaries.
Arterial Supply to the Kidney
The kidney’s blood supply is disproportionately high compared to that of other organs. Although the kidneys represent only 1 % of the body mass, they receive 25 % of the cardiac output, five times the blood flow through the coronary arteries [15]. Since only 1 % of the glomerular filtrate is normally excreted as urine, there is a comparably impressive venous return that exits the renal sinus through the renal veins . Thus, tumors arising within the kidney are heavily perfused with a voluminous venous return that potentiates venous metastases.
One of the first observations in a nephrectomy for RCC is examination of the vascular hilum, and in particular, assessment of the hilar vessels. Although there are countless variations in the organization of the renal arteries (and veins as discussed below), there are a few generalizations that apply to most nephrectomy specimens [3, 11–14]. The most common arterial arrangement is for the main renal artery to give rise to an anterior and posterior division. Four segmental arteries arise from the anterior division to supply the upper and lower poles and the anterior kidney. The posterior division continues as the posterior segmental artery. The segmental arteries sequentially branch into the interlobar arteries that enter the renal parenchyma giving rise to six to eight arcuate arteries, from which the interlobular arteries are derived that ascend to the renal capsule. These arteries are all end arteries. There is a vascular junction between the anterior and posterior blood supply 1–2 cm posterior to the lateral convex border of the kidney. This is known as “Brödel’s bloodless line of incision,” a useful landmark for surgical entry to the kidney [11] .
Although the arteries play no role in tumor dissemination or staging, documentation of significant atherosclerotic disease is important because of its role in nephrosclerosis. The major grossing issue related to renal arteries is their frequent tendency to intertwine with the major tributaries of the main renal vein at the renal hilum (Fig. 22.8). This complicates the dissection of the renal veins and may explain the all too frequent occurrence of stapling of arteries and veins together, especially with laparoscopic resections .
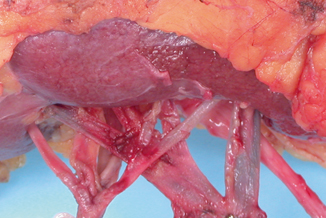

Fig. 22.8




This image shows the vascular hilum of a left kidney. Notice that the arteries and vein are located anterior to the renal pelvis. The artery on the left has five segmental branches. The main renal vein on the right is formed by the confluence of four tributaries. Notice that the segmental renal arteries and veins interdigitate
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


