40
Aminoglycosides
This chapter will be most useful after having a basic understanding of the material in Chapter 54, Aminoglycosides in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition contains:
• Figure 54-1 which shows the sites of activity of various plasmid-mediated enzymes capable of inactivating aminoglycosides
• Table 54-1 which provides the minimal inhibitory concentrations of aminoglycosides that will inhibit 90% (MIC90) of clinical isolates for several bacterial species
• Table 54-2 which provides an algorithm for dose reduction of aminoglycosides based on creatinine clearance
LEARNING OBJECTIVES
 Understand aminoglycoside mechanisms of action and resistance.
Understand aminoglycoside mechanisms of action and resistance.
 Describe the advantages and disadvantages of multiple daily dosing versus once daily extended-interval dosing regimens for aminoglycosides.
Describe the advantages and disadvantages of multiple daily dosing versus once daily extended-interval dosing regimens for aminoglycosides.
 Describe the rationale and the methods of plasma concentration monitoring of aminoglycoside therapy.
Describe the rationale and the methods of plasma concentration monitoring of aminoglycoside therapy.
 Describe the causes and clinical signs of aminoglycoside ototoxicity and nephrotoxicity and the best means of monitoring therapy to avoid these serious toxicities.
Describe the causes and clinical signs of aminoglycoside ototoxicity and nephrotoxicity and the best means of monitoring therapy to avoid these serious toxicities.
 Understand the unique clinical differences among the aminoglycosides.
Understand the unique clinical differences among the aminoglycosides.
The mechanisms of action and resistance of the aminoglycosides are shown in the side bars MECHANISMS OF ACTION OF AMINOGLYCOSIDE and MECHANISMS OF AMINOGLYCOSIDE RESISTANCE, respectively.
DRUGS INCLUDED IN THIS CHAPTER
• Amikacin
• Gentamicin (GARAMYCIN, others)
• Kanamycin
• Neomycin
• Netilmicin (NETROMYCIN)
• Streptomycin
• Tobramycin (TOBREX, others)
MECHANISMS OF ACTION OF AMINOGLYCOSIDES
• The aminoglycosides are rapidly bactericidal and their bacterial killing is concentration-dependent
• Aminoglycosides exhibit a postantibiotic effect, that is, the bactericidal activity persists after serum concentration falls below the MIC
• Duration of postantibiotic effect is also concentration-dependent
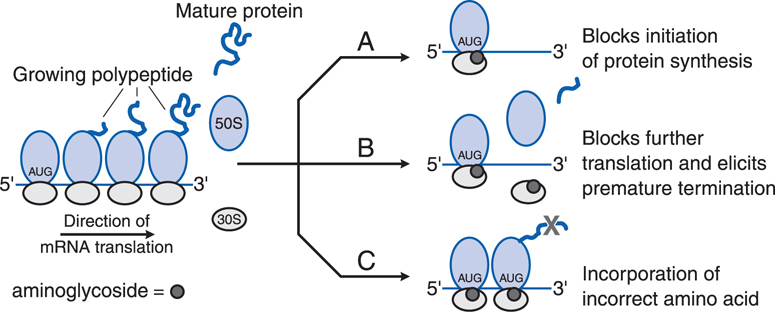
• Inside the bacterial cell, the aminoglycosides bind to polysomes and interfere with protein synthesis by causing misreading and premature termination of mRNA translation (see Figure 40-1)
FIGURE 40-1 Effects of aminoglycosides on protein synthesis. A. Aminoglycoside (represented by dark grey circles) binds to the 30S ribosomal subunit and interferes with initiation of protein synthesis by fixing the 30S to 50S ribosomal complex at the start codon (AUG) of mRNA. As 30S to 50S complexes downstream complete translation of mRNA and detach, the abnormal initiation complexes, the so-called streptomycin monosomes, accumulate, blocking further translation of the message. Aminoglycoside binding to the 30S subunit also causes misreading of mRNA, leading to B, premature termination of translation with detachment of the ribosomal complex and incompletely synthesized protein or C, incorporation of incorrect amino acids (indicated by the grey X), resulting in the production of abnormal or nonfunctional proteins.
MECHANISMS OF AMINOGLYCOSIDE RESISTANCE
• Failure of the aminoglycoside to penetrate the bacteria cell
• Inactivation of the aminoglycoside by microbial enzymes
• Low affinity of the aminoglycoside for the bacterial ribosome
A 56-year-old woman is in hospital for the treatment of pneumonia. Her case is complicated because she acquired the pneumonia in another hospital while undergoing post-surgical rehabilitation for a hysterectomy. She was being treated with a cephalosporin at the other hospital, but gentamicin is now added.
a. Why was the aminoglycoside added to her therapeutic regimen?
An aminoglycoside in combination with a β-lactam antibiotic is recommended as standard therapy for hospital-acquired pneumonia in which a multiple-drug resistant gram-negative aerobe is a likely causative agent. If it is established that the β-lactam is active against the causative agent, there is generally no need to continue the aminoglycoside.
b. Why is the combination of a β-lactam antibiotic and an aminoglycoside effective?
These 2 antibiotic classes have distinctly different mechanisms of action and their effectiveness should be complementary. β-Lactam antibiotics inhibit cell wall synthesis (see Chapter 39). Aminoglycosides act at the 30S ribosomal subunit to disrupt the normal cycle of ribosomal function by interfering with the initiation of protein synthesis, also by leading to accumulation of abnormal initiation complexes and premature termination, and by causing misreading of the mRNA template and incorporation of incorrect amino acids into the growing polypeptide chains (see Figure 40-1).
A 23-year-old woman is being treated with gentamicin for an enterococci infection. Twenty-four hours after beginning treatment it is learned that the organism is resistant to gentamicin.
a. Does resistance to gentamicin mean resistance to other aminoglycosides?
Resistance to gentamicin indicates cross-resistance to tobramycin, amikacin, kanamycin, and netilmicin because the inactivating enzyme is bifunctional and can modify all these aminoglycosides. The chemical structure of streptomycin is sufficiently different from gentamicin that this inactivating enzyme does not modify streptomycin; consequently gentamicin-resistant strains of enterococci may be susceptible to streptomycin.
Amikacin is a suitable substrate for only a few of the inactivating enzymes that confer gentamicin-resistance; thus, strains that are resistant to multiple other aminoglycosides tend to be susceptible to amikacin.
A significant percentage of clinical isolates of Enterococcus faecalis and E. faecium are highly resistant to all aminoglycosides.
b. What are the likely mechanisms of aminoglycoside resistance?
See Side Bar MECHANISMS OF AMINOGLYCOSIDE RESISTANCE. Clinically, drug inactivation is the most common mechanism for acquired microbial resistance to aminoglycosides. The genes encoding aminoglycoside-modifying enzymes are acquired primarily by conjugation and transfer of resistance plasmids. These enzymes phosphorylate, adenylate, or acetylate specific hydroxyl or amino groups.
Other mechanisms of aminoglycoside resistance include the inability of the aminoglycoside to penetrate bacterial intracellular space and a reduced affinity of the aminoglycoside for the bacterial ribosome.
A 65-year-old man is in hospital for the treatment of community-acquired pneumonia that is sensitive to an aminoglycoside. He is being treated with 5.1 mg/kg of gentamicin as a single dose once every 24 hours.
a. Based on a half-life of 2 to 3 hours, aminoglycosides have been historically administered in 3 equally divided doses over a 24-hour period. Is this patient’s single dose expected to be effective?
Numerous studies and meta-analyses demonstrate that administration of the total dose of aminoglycoside once daily is just as effective as multiple-dose regimens. In addition, extended-interval administration costs less, is administered more easily, and is associated with less nephrotoxicity.
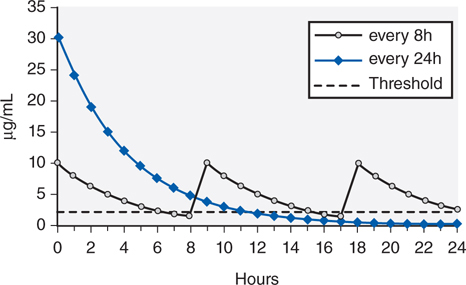
A comparison of high-dose, extended-interval dosing to traditional divided-dose methods is illustrated in Figure 40-2. Because of the postantibiotic effect of aminoglycosides, good therapeutic response can be attained even when the concentrations of aminoglycosides fall below bacterial inhibitory concentrations for a substantial period of the dosing interval.
FIGURE 40-2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree