Aminoglycosides
Aminoglycosides (gentamicin, tobramycin, amikacin, netilmicin, kanamycin, streptomycin, paromomycin, and neomycin) are used primarily to treat infections caused by aerobic gram-negative bacteria. Streptomycin is an important agent for the treatment of tuberculosis, and paromomycin is used orally for intestinal amebiasis and in the management of hepatic coma. Aminoglycosides are bactericidal inhibitors of protein synthesis. Mutations affecting proteins in the bacterial ribosome can confer marked resistance to their action. Most commonly resistance is due to acquisition of plasmids or transposon-encoding genes for aminoglycoside-metabolizing enzymes or from impaired transport of drug into the cell. Thus, there can be cross-resistance between members of the class.
Aminoglycosides are natural products or semisynthetic derivatives of compounds produced by a variety of soil actinomycetes. Amikacin, a derivative of kanamycin, and netilmicin, a derivative of sisomicin, are semisynthetic products. These agents contain amino sugars linked to an aminocyclitol ring by glycosidic bonds (Figure 54–1). They are polycations, and their polarity is responsible in part for pharmacokinetic properties shared by all members of the group. For example, none is absorbed adequately after oral administration, inadequate concentrations are found in cerebrospinal fluid (CSF), and all are excreted relatively rapidly by the normal kidney. All members of the group share the same spectrum of toxicity, most notably nephrotoxicity and ototoxicity, which can involve the auditory and vestibular functions of the eighth cranial nerve.
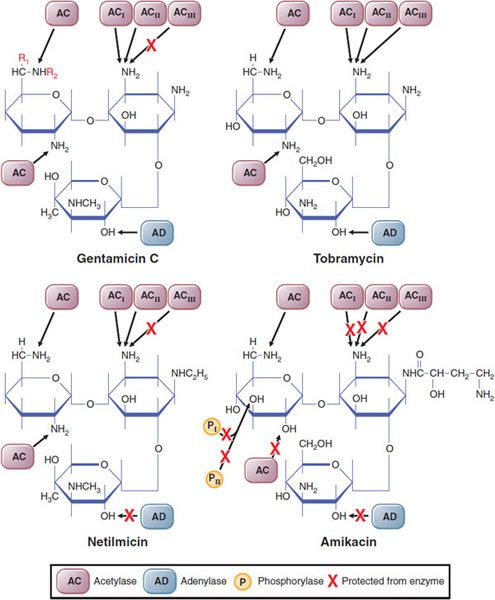
Figure 54–1 Sites of activity of various plasmid-mediated enzymes capable of inactivating aminoglycosides. The red X indicates regions of the molecules that are protected from the designated enzyme. In gentamicin C1, R1R2CH3; in gentamicin C2, R1CH3, R2H; in gentamicin C1a, R1R2H. (Reproduced with permission from Moellering RC Jr. Microbiological considerations in the use of tobramycin and related aninoglycosidic aminocyclitol antibiotics. Med J Aust, 1977;2S:4–8. Copyright 1977. The Medical Journal of Australia.)
GENERAL PROPERTIES
MECHANISM OF ACTION. The aminoglycoside antibiotics are rapidly bactericidal. Bacterial killing is concentration dependent: the higher the concentration, the greater the rate of bacterial killing. The bactericidal activity persists after the serum concentration has fallen below the minimum inhibitory concentration (MIC). These properties probably account for the efficacy of high-dose, extended-interval dosing regimens.
Aminoglycosides diffuse through aqueous channels formed by porin proteins in the outer membrane of gram-negative bacteria to enter the periplasmic space. Transport of aminoglycosides across the cytoplasmic (inner) membrane depends on a transmembrane electrical gradient coupled to electron transport to drive permeation of these antibiotics. This energy-dependent phase is rate-limiting and can be blocked or inhibited by divalent cations (e.g., Ca2+ and Mg2+), hyperosmolarity, a reduction in pH, and anaerobic conditions. Thus, the antimicrobial activity of aminoglycosides is reduced markedly in the anaerobic environment of an abscess and in hyperosmolar acidic urine.
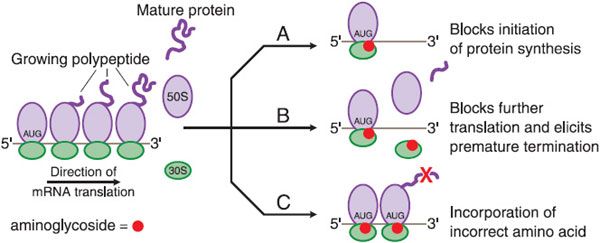
Once inside the cell, aminoglycosides bind to polysomes and interfere with protein synthesis by causing misreading and premature termination of mRNA translation (Figure 54–2). The primary intracellular site of action of the aminoglycosides is the 30S ribosomal subunit. At least 3 of these ribosomal proteins, and perhaps the 16S ribosomal RNA as well, contribute to the streptomycin-binding site. Aminoglycosides interfere with the initiation of protein synthesis, leading to the accumulation of abnormal initiation complexes; the drugs also can cause misreading of the mRNA template and incorporation of incorrect amino acids into the growing polypeptide chains. The resulting aberrant proteins may be inserted into the cell membrane, leading to altered permeability and further stimulation of aminoglycoside transport.
Figure 54–2 Effects of aminoglycosides on protein synthesis. A. Aminoglycoside (represented by red circles) binds to the 30S ribosomal subunit and interferes with initiation of protein synthesis by fixing the 30S-50S ribosomal complex at the start codon (AUG) of mRNA. As 30S-50S complexes downstream complete translation of mRNA and detach, the abnormal initiation complexes, so-called streptomycin monosomes, accumulate, blocking further translation of the message. Aminoglycoside binding to the 30S subunit also causes misreading of mRNA, leading to B, premature termination of translation with detachment of the ribosomal complex and incompletely synthesized protein or C, incorporation of incorrect amino acids (indicated by the red X), resulting in the production of abnormal or nonfunctional proteins.
MICROBIAL RESISTANCE TO THE AMINOGLYCOSIDES. Bacteria may be resistant to aminoglycosides because of:
• Inactivation of the drug by microbial enzymes
• Failure of the antibiotic to penetrate intracellularly
• Low affinity of the drug for the bacterial ribosome
Clinically, drug inactivation is the most common mechanism for acquired microbial resistance. The genes encoding aminoglycoside-modifying enzymes are acquired primarily by conjugation and transfer of resistance plasmids (see Chapter 48). These enzymes phosphorylate, adenylate, or acetylate specific hydroxyl or amino groups (see Figure 54–1). Amikacin is a suitable substrate for only a few of these inactivating enzymes (see Figure 54–1); thus, strains that are resistant to multiple other aminoglycosides tend to be susceptible to amikacin. However, a significant percentage of clinical isolates of Enterococcus faecalis and E. faecium are highly resistant to all aminoglycosides. Resistance to gentamicin indicates cross-resistance to tobramycin, amikacin, kanamycin, and netilmicin because the inactivating enzyme is bifunctional and can modify all these aminoglycosides. Owing to differences in the chemical structures of streptomycin and other aminoglycosides, this enzyme does not modify streptomycin, which is inactivated by another enzyme; consequently, gentamicin-resistant strains of enterococci may be susceptible to streptomycin. Intrinsic resistance to aminoglycosides may be caused by failure of the drug to penetrate the cytoplasmic (inner) membrane. Transport of aminoglycosides across the cytoplasmic membrane is an active process that depends on oxidative metabolism. Strictly anaerobic bacteria thus are resistant to these drugs because they lack the necessary transport system.
Missense mutations in Escherichia coli that substitute a single amino acid in a crucial ribosomal protein may prevent binding of streptomycin. Although highly resistant to streptomycin, these strains are not widespread in nature. Similarly, 5% of strains of Pseudomonas aeruginosa exhibit such ribosomal resistance to streptomycin. Because ribosomal resistance usually is specific for streptomycin, these strains of enterococci remain sensitive to a combination of penicillin and gentamicin in vitro.
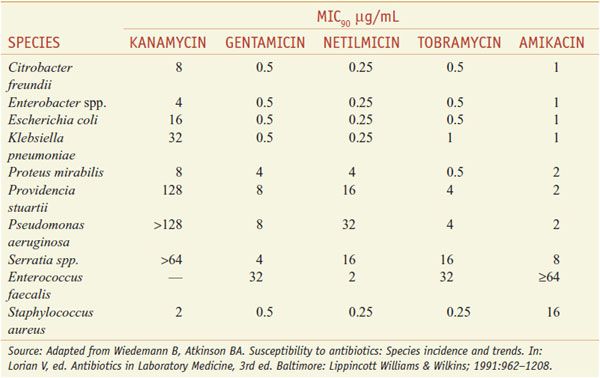
ANTIBACTERIAL SPECTRUM OF THE AMINOGLYCOSIDES. The antibacterial activity of gentamicin, tobramycin, kanamycin, netilmicin, and amikacin is directed primarily against aerobic gram-negative bacilli. Kanamycin, like streptomycin, has a more limited spectrum. The aerobic gram-negative bacilli vary in their susceptibility to the aminoglycosides (see Table 54–1).
Table 54–1
Typical Minimal Inhibitory Concentrations of Aminoglycosides That Will Inhibit 90% (MIC90) of Clinical Isolates for Several Species
Aminoglycosides have little activity against anaerobic microorganisms or facultative bacteria under anaerobic conditions. Their action against most gram-positive bacteria is limited, and they should not be used as single agents to treat infections caused by gram-positive bacteria. In combination with a cell wall–active agent, such as a penicillin or vancomycin, an aminoglycoside produces a synergistic bactericidal effect in vitro. Clinically, the superiority of aminoglycoside combination regimens over β-lactams alone is not proven except in relatively few infections (discussed later).
ADME AND DOSING
ABSORPTION. The aminoglycosides are polar cations and therefore are poorly absorbed from the GI tract. Less than 1% of a dose is absorbed after either oral or rectal administration. The drugs are eliminated quantitatively in the feces. Nonetheless, long-term oral or rectal administration of aminoglycosides may result in accumulation to toxic concentrations in patients with renal impairment. Absorption of gentamicin from the GI tract may be increased by GI disease (e.g., ulcers or inflammatory bowel disease). Instillation of these drugs into body cavities with serosal surfaces also may result in rapid absorption and unexpected toxicity (i.e., neuromuscular blockade). Intoxication may occur when aminoglycosides are applied topically for long periods to large wounds, burns, or cutaneous ulcers, particularly if there is renal insufficiency.
All the aminoglycosides are absorbed rapidly from intramuscular sites of injection. Peak concentrations in plasma occur after 30-90 min. These concentrations range from 4-12 μg/mL following a 1.5-2 mg/kg dose of gentamicin, tobramycin, or netilmicin and from 20-35 μg/mL following a 7.5 mg/kg dose of amikacin or kanamycin. There is increasing use of aminoglycosides administered via inhalation, primarily for the management of patients with cystic fibrosis who have chronic P. aeruginosa pulmonary infections. Amikacin and tobramycin solutions for injection have been used, as well as a commercial formulation of tobramycin designed for inhalation (TOBI, others).
DISTRIBUTION. Because of their polar nature, the aminoglycosides do not penetrate into most cells, the CNS, or the eye. Except for streptomycin, there is negligible binding of aminoglycosides to plasma albumin. The apparent volume of distribution of these drugs is 25% of lean body weight and approximates the volume of extracellular fluid. The aminoglycosides distribute poorly into adipose tissue, which must be considered when using weight-based dosing regimens in obese patients.
Concentrations of aminoglycosides in secretions and tissues are low. High concentrations are found only in the renal cortex and the endolymph and perilymph of the inner ear; the high concentration in these sites likely contribute to the nephrotoxicity and ototoxicity caused by these drugs. As a result of active hepatic secretion, concentrations in bile approach 30% of those found in plasma, but this represents a very minor excretory route for the aminoglycosides. Inflammation increases the penetration of aminoglycosides into peritoneal and pericardial cavities. Concentrations of aminoglycosides achieved in CSF with parenteral administration usually are subtherapeutic. Treatment of meningitis with intravenous administration is generally suboptimal. Intrathecal or intraventricular administration of aminoglycosides has been used to achieve therapeutic levels, but the availability of third- and fourth-generation cephalosporins has generally made this unnecessary.
Administration of aminoglycosides to women late in pregnancy may result in accumulation of drug in fetal plasma and amniotic fluid. Streptomycin and tobramycin can cause hearing loss in children born to women who receive the drug during pregnancy. Insufficient data are available regarding the other aminoglycosides; therefore, these agents should be used with caution during pregnancy and only for strong clinical indications in the absence of suitable alternatives.
ELIMINATION. The aminoglycosides are excreted almost entirely by glomerular filtration, achieving urine concentrations of 50-200 μg/mL. The half-lives of the aminoglycosides in plasma are 2-3 h in patients with normal renal function. Because the elimination of aminoglycosides depends almost entirely on the kidney, a linear relationship exists between the concentration of creatinine in plasma and the t1/2 of all aminoglycosides in patients with moderately compromised renal function. Because the incidence of nephrotoxicity and ototoxicity is likely related to the overall drug exposure to aminoglycosides, it is critical to reduce the maintenance dosage of these drugs in patients with impaired renal function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree