Objectives
- Defines alveolar ventilation.
- Defines the standard lung volumes and understands their measurement.
- Predicts the effects of alterations in lung and chest wall mechanics, due to normal or pathologic processes, on the lung volumes.
- Defines anatomic dead space and relates the anatomic dead space and the tidal volume to alveolar ventilation.
- Understands the measurement of the anatomic dead space and the determination of alveolar ventilation.
- Defines physiologic and alveolar dead space and understands their determination.
- Predicts the effects of alterations of alveolar ventilation on alveolar carbon dioxide and oxygen levels.
- Describes the regional differences in alveolar ventilation found in the normal lung and explains these differences.
- Predicts the effects of changes in lung volume, aging, and disease processes on the regional distribution of alveolar ventilation.
- Defines the closing volume and explains how it can be demonstrated.
- Predicts the effects of changes in pulmonary mechanics on the closing volume.
Alveolar Ventilation: Introduction
Alveolar ventilation is the exchange of gas between the alveoli and the external environment. It is the process by which oxygen is brought into the lungs from the atmosphere and by which the carbon dioxide carried into the lungs in the mixed venous blood is expelled from the body. Although alveolar ventilation is usually defined as the volume of fresh air entering the alveoli per minute, a similar volume of alveolar air leaving the body per minute is implicit in this definition.
The Lung Volumes
The volume of gas in the lungs at any instant depends on the mechanics of the lungs and chest wall and the activity of the muscles of inspiration and expiration. The lung volume under any specified set of conditions can be altered by pathologic and normal physiologic processes. Standardization of the conditions under which lung volumes are measured allows comparisons to be made among subjects or patients. The size of a person’s lungs depends on his or her height and weight or body surface area, as well as on his or her age and sex. Therefore, the lung volumes for a patient are usually compared with data in a table of “predicted” lung volumes matched to age, sex, and body size. The lung volumes are normally expressed at the Body Temperature and ambient Pressure and Saturated with water vapor (BTPS).
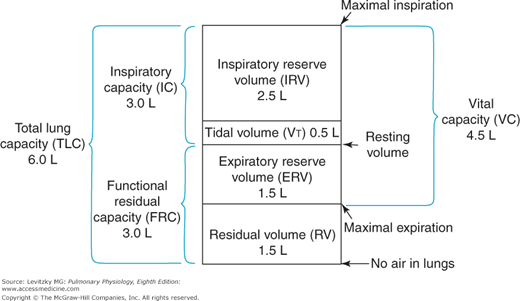
There are 4 standard lung volumes (which are not subdivided) and 4 standard lung capacities, which consist of 2 or more standard lung volumes in combination (Figure 3–1).
The tidal volume (VT) is the volume of air entering or leaving the nose or mouth per breath. It is determined by the activity of the respiratory control centers in the brain as they affect the respiratory muscles and by the mechanics of the lung and the chest wall. During normal, quiet breathing (eupnea) the VT of a 70-kg adult is about 500 mL per breath, but this volume can increase dramatically, for example, during exercise.
The residual volume (RV) is the volume of gas left in the lungs after a maximal forced expiration. It is determined by the force generated by the muscles of expiration and the inward elastic recoil of the lungs as they oppose the outward elastic recoil of the chest wall. Dynamic compression of the airways during the forced expiratory effort may also be an important determinant of the RV as airway collapse occurs, thus trapping gas in the alveoli. The RV of a healthy 70-kg adult is about 1.5 L, but it can be much greater in a disease state such as emphysema, in which inward alveolar elastic recoil is diminished and much airway collapse and gas trapping occur. The RV is important to a healthy person because it prevents the lungs from collapsing at very low lung volumes. The collapsed alveoli would require very great inspiratory efforts to reinflate.
The expiratory reserve volume (ERV) is the volume of gas that is expelled from the lungs during a maximal forced expiration that starts at the end of a normal tidal expiration. It is therefore determined by the difference between the functional residual capacity (FRC) and the RV. The ERV is about 1.5 L in a healthy 70-kg adult.
The inspiratory reserve volume (IRV) is the volume of gas that is inhaled into the lungs during a maximal forced inspiration starting at the end of a normal tidal inspiration. It is determined by the strength of contraction of the inspiratory muscles, the inward elastic recoil of the lung and the chest wall, and the starting point, which is the FRC plus the VT. The IRV of a normal 70-kg adult is about 2.5 L.
The FRC is the volume of gas remaining in the lungs at the end of a normal tidal expiration. Because it was traditionally assumed that no muscles of respiration are contracting at the end of a normal tidal expiration, the FRC is usually considered to represent the balance point between the inward elastic recoil of the lungs and the outward elastic recoil of the chest wall, as discussed in Chapter 2.
However, the respiratory muscles may have significant tone at the FRC, and in certain circumstances, the FRC may be greater than or even less than the lung volume of the totally relaxed respiratory system. Thus, the lung volume at which the inward elastic recoil of the lungs is equal and opposite to the outward elastic recoil of the chest wall is sometimes referred to as the relaxation volume of the respiratory system. The FRC may be greater than the relaxation volume if the next inspiration occurs before the relaxation volume is reached, either because of high breathing rates or high resistance to expiratory airflow in the larynx or peripheral airways, or because of active contraction of the inspiratory muscles at end expiration. Either or both of these may occur in babies, who have higher FRCs than would be predicted from the great inward elastic recoil of their lungs and the small outward recoil of their chest walls. During exercise, the FRC may be lower than the relaxation volume because of active contraction of the expiratory muscles.
The FRC, as seen in Figure 3–1, consists of the RV plus the ERV. It is therefore about 3 L in a healthy 70-kg adult.
The inspiratory capacity (IC) is the volume of air that is inhaled into the lungs during a maximal inspiratory effort that begins at the end of a normal tidal expiration (the FRC). It is therefore equal to the VT plus the IRV, as shown in Figure 3–1. The IC of a normal 70-kg adult is about 3 L.
The total lung capacity (TLC) is the volume of air in the lungs after a maximal inspiratory effort. It is determined by the strength of contraction of the inspiratory muscles and the inward elastic recoil of the lungs and the chest wall. The TLC consists of all 4 lung volumes: the RV, the VT, the IRV, and the ERV. The TLC is about 6 L in a healthy 70-kg adult.
The vital capacity (VC), discussed in Chapter 2, is the volume of air expelled from the lungs during a maximal forced expiration starting after a maximal forced inspiration. The VC is therefore equal to the TLC minus the RV, or about 4.5 L in a healthy 70-kg adult. The VC is also equal to the sum of the VT and the IRV and ERV. It is determined by the factors that determine the TLC and RV.
Measurement of the Lung Volumes
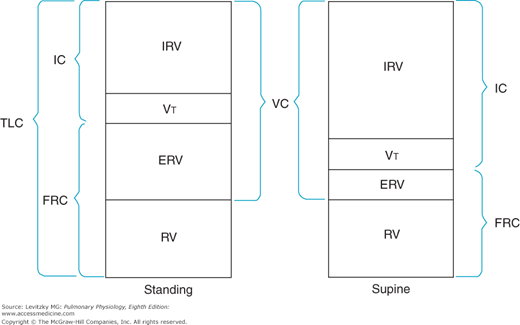
Measurement of the lung volumes is important clinically because many pathologic states can alter specific lung volumes or their relationships to one another. The lung volumes, however, can also change for normal physiologic reasons. Changing from a standing to a supine posture decreases the FRC because gravity is no longer pulling the abdominal contents away from the diaphragm. This decreases the outward elastic recoil of the chest wall, as noted in Chapter 2, Figure 2–14. The RV and TLC do not change significantly when a person changes from standing to the supine position. If the FRC is decreased, then the ERV will also decrease (Figure 3–2), and the IRV will increase. The VC, RV, and TLC may decrease slightly because some of the venous blood that collects in the lower extremities and the abdomen when a person is standing returns to the thoracic cavity when that person lies down.
Figure 3–2.
Illustration of alterations in the lung volumes and capacities that occur when a subject changes from the standing to the supine position. IC = inspiratory capacity; TLC = total lung capacity; FRC = functional residual capacity; IRV = inspiratory reserve volume; VT = tidal volume; ERV = expiratory reserve volume; RV = residual volume; VC = vital capacity.
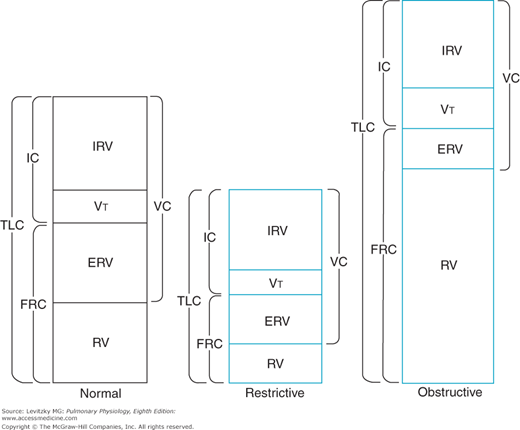
Determination of the lung volumes can be useful diagnostically in differentiating between 2 major types of pulmonary disorders—the restrictive diseases and the obstructive diseases. Restrictive diseases like alveolar fibrosis, which reduce the compliance of the lungs, lead to compressed lung volumes (Figure 3–3). The increased elastic recoil of the lungs leads to a lower FRC, a lower TLC, a lower VC, and lower IRV and ERV, and it may even decrease the RV. The VT may also be decreased, with a corresponding increase in breathing frequency, to minimize the work of breathing.
Figure 3–3.
Illustration of typical alterations in the lung volumes and capacities in restrictive and obstructive diseases. The pattern shown for obstructive diseases is more characteristic for emphysema and asthma than for chronic bronchitis. IC = inspiratory capacity; TLC = total lung capacity; FRC = functional residual capacity; IRV = inspiratory reserve volume; VT = tidal volume; ERV = expiratory reserve volume; RV = residual volume; VC = vital capacity.
Obstructive diseases such as emphysema and chronic bronchitis cause increased resistance to airflow. Airways may become completely obstructed because of mucus plugs as well as because of the high intrapleural pressures generated to overcome the elevated airways resistance during a forced expiration. This is especially a problem in emphysema, in which destruction of alveolar septa leads to decreased elastic recoil of the alveoli and less radial traction, which normally help hold small airways open. For these reasons, the RV, the FRC, and the TLC may be greatly increased in obstructive diseases, as seen in Figure 3–3. The VC and ERV are usually decreased. The breathing frequency may be decreased to reduce the work expended overcoming the airways resistance, with a corresponding increase in the VT.
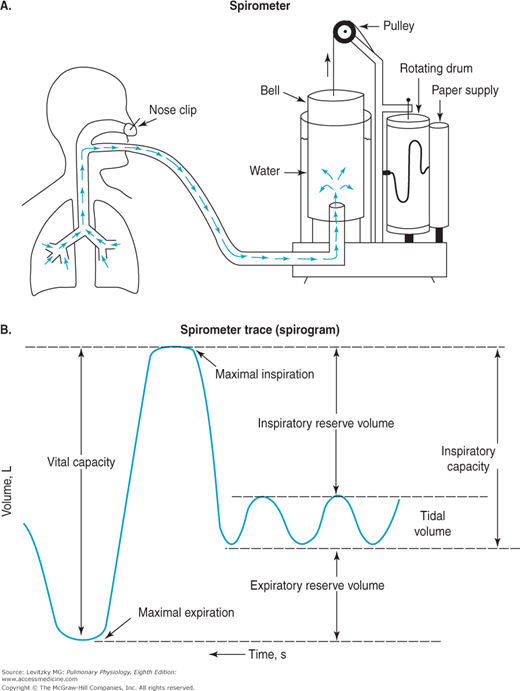
The spirometer is a simple device for measuring gas volumes. The traditionally used water sealed spirometer, shown in Figure 3–4, consists of an inverted canister, or “bell,” floating in a water-filled space between 2 concentrically arranged cylinders. The space inside the inner drum, which is closed off from the atmosphere by the bell, is connected to tubing that extends to a mouthpiece into which the person breathes. As the person breathes in and out, gas enters and leaves the spirometer, and the bell then floats higher (during expiration) and lower (during inspiration). The top of the bell is connected by a pulley to a pen that writes on a rotating drum, thus tracing the person’s breathing pattern.
As is evident from Figure 3–4, the spirometer can measure only the lung volumes that the subject can exchange with it. As is the case with many pulmonary function tests, the subject must be conscious and cooperative and understand the instructions for performing the test. The VT, IRV, ERV, IC, and VC can all be measured with a spirometer (as can the forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], and forced expiratory flow [FEF25%–75%], as discussed in Chapter 2). The RV, the FRC, and the TLC, however, cannot be determined with a spirometer because the subject cannot exhale all the gas in the lungs. The gas in a spirometer is at Ambient Temperature, Pressure, and water vapor Saturation (ATPS), and the volumes of gas collected in a spirometer must be converted to equivalent volumes in the body. Other kinds of spirometers include rolling seal and bellows spirometers. These spirometers are not water-filled and are more portable.
The lung volumes not measurable with spirometry can be determined by the nitrogen-washout technique, by the helium-dilution technique, and by body plethysmography. The FRC is usually determined, and RV (which is equal to FRC minus ERV) and the TLC (which is equal to VC plus RV) are then calculated from volumes obtained by spirometry.
In the nitrogen-washout technique, the person breathes 100% oxygen through a 1-way valve so that all the expired gas is collected. The concentration of nitrogen in the expired air is monitored with a nitrogen analyzer until it reaches zero. At this point, all the nitrogen is theoretically washed out of the person’s lungs. The total volume of all the gas the person expired is determined, and this amount is multiplied by the percentage of nitrogen in the mixed expired air, which can be determined with the nitrogen analyzer. The total volume of nitrogen in the person’s lungs at the beginning of the test can thus be determined. Nitrogen constitutes about 80% of the person’s initial lung volume, and so multiplying the initial nitrogen volume by 1.25 gives the person’s initial lung volume. If the test is begun at the end of a normal tidal expiration, the volume determined is the FRC:
Total volume expired × % N2 = original volume of N2 in lungs
Original volume of N2 in lungs × 1.25 = original lung volume
The helium-dilution technique, like other indicator dilution techniques, makes use of the following relationship: If the total amount of a substance dissolved in a volume is known and its concentration can be measured, the volume in which it is dissolved can be determined. For example, if a known amount of a solute is dissolved in an unknown volume of solvent, and the concentration of the solute can be determined, then the volume of solvent can be calculated:
Amount of solute (mg) = concentration of solute (mg/mL) × volume of solvent (mL)
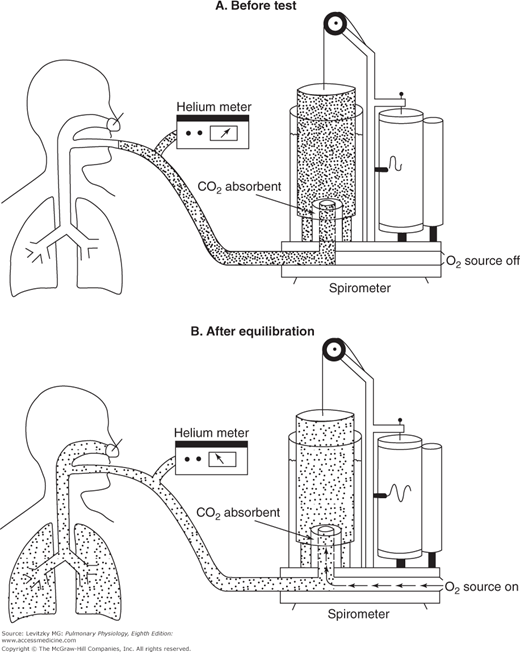
In the helium-dilution technique, helium is dissolved in the gas in the lungs and its concentration is determined with a helium meter, allowing calculation of the lung volume. Helium is used for this test because it is not taken up by the pulmonary capillary blood and because it does not diffuse out of the blood, and so the total amount of helium does not change during the test. The person breathes in and out of a spirometer filled with a mixture of helium and oxygen, as shown in Figure 3–5. The helium concentration is monitored continuously with a helium meter until its concentration in the inspired air equals its concentration in the person’s expired air. At this point, the concentration of helium is the same in the person’s lungs as it is in the spirometer, and the test is stopped at the end of a normal tidal expiration, in other words, at the FRC.
Figure 3–5.
The helium-dilution technique for the determination of the functional residual capacity. A: Before the test, the spirometer is filled with a mixture of helium (denoted by the dots) and oxygen. The concentration of helium is determined by the helium meter. B: The subject breathes from the spirometer until the helium concentration in the lungs equilibrates with that in the spirometer. During the equilibration period, the subject’s expired carbon dioxide is absorbed and oxygen is added to the spirometer at the subject’s oxygen consumption rate. The helium concentration and spirometer volume are determined after equilibration, when the subject is at functional residual capacity.
The FRC can then be determined by the following formula (total amount of He before test = total amount of He at end of test):
FHEi Vspi = FHEf (Vspf + VLf)
That is, the total amount of helium in the system initially is equal to its initial fractional concentration (Fhei) times the initial volume of the spirometer (Vspi). This must be equal to the total amount of helium in the lungs and the spirometer at the end of the test, which is equal to the final (lower) fractional concentration of helium (Fhef) times the final volume of the spirometer (Vspf) and the volume of the lungs at the end of the test (Vlf). Since it may take several minutes for the helium concentration to equilibrate between the lungs and the spirometer, in practice, CO2 is absorbed from the system and oxygen is added to the spirometer at the rate at which it is consumed by the person. A correction factor is used to account for the small amount of helium that does dissolve in the blood during the test. Both the nitrogen-washout and helium-dilution methods can be used on unconscious patients.
A problem common to both the nitrogen-washout technique and the helium-dilution technique is that neither can measure trapped gas: the nitrogen trapped in alveoli supplied by closed airways cannot be washed out and the helium cannot enter alveoli supplied by closed airways. Furthermore, if the patient’s lungs have many alveoli served by airways with high resistance to airflow (the “slow alveoli” discussed at the end of Chapter 2), it may take a very long time for all the nitrogen to wash out of the patient’s lungs or for the inspired and expired helium concentrations to equilibrate. In such patients, measurements of the lung volumes with a body plethysmograph are much more accurate because they do include trapped gas.
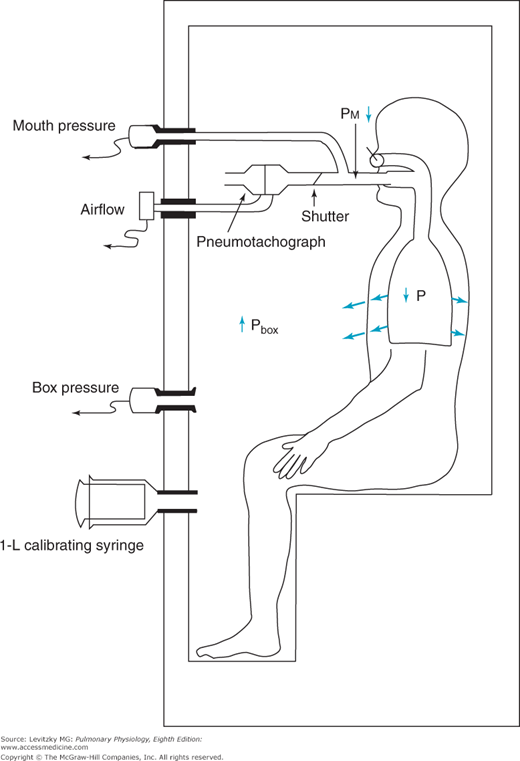
The body plethysmograph makes use of Boyle’s law, which states that for a closed container at a constant temperature, the pressure times the volume is constant. The body plethysmograph, an expensive piece of equipment, is shown schematically in Figure 3–6.
Figure 3–6.
The use of the body plethysmograph for the determination of the functional residual capacity. The subject is seated in the small airtight chamber and breathes through the apparatus shown. By monitoring the subject’s airflow with a pneumotachograph, the operator can briefly occlude the subject’s airway at end expiration. As the subject makes an inspiratory effort against the closed airway, the pressure in the chamber (Pbox) increases and the pressure at the subject’s mouth (Pm) decreases. The subject’s functional residual capacity can then be calculated.
As can be seen from the figure, the body plethysmograph is an airtight chamber large enough so that the patient can sit inside it. The patient sits in the closed plethysmograph, or “box,” and breathes through a mouthpiece and tubing. The tubing contains a sidearm connected to a pressure transducer (“mouth pressure”), an electrically controlled shutter that can occlude the airway when activated by the person conducting the test, and a pneumotachograph to measure airflow, allowing the operator to follow the subject’s breathing pattern. A second pressure transducer, which must be very sensitive, monitors the pressure in the plethysmograph (“box pressure”).
After the subject breathes through the open tube for a while to establish a normal breathing pattern, the operator closes the shutter in the airway at the end of a normal tidal expiration. At this point, the subject breathes in for an instant against a closed airway. As the subject breathes in against the closed airway, the chest continues to expand and the pressure measured by the transducer in the plethysmograph (Pbox) increases because the volume of air in the plethysmograph (Vbox) decreases by the amount the patient’s chest volume increased (ΔV):
Pboxi × Vboxi = Pboxf × (Vboxi − ΔV) (1)
where (Vboxi – ΔV) = Vboxf
That is, the product of the initial box pressure times the initial box volume must equal the final box pressure times the final box volume (the initial box volume minus a change in volume), according to Boyle’s law. Of course, direct measurement of box volume, which is really equal to the volume of the plethysmograph minus the volume occupied by the patient, is impossible, and so the plethysmograph is calibrated by injecting known volumes of air into the plethysmograph and determining the increase in pressure. After such a graph of pressure changes with known changes in volume has been constructed, the ΔV in Equation (1) can be determined.
The product of the pressure measured at the mouth (Pm) times the volume of the patient’s lungs (Vl) must also be constant during the inspiration against a closed airway. As the patient breathes in, the volume of the lungs increases by the same amount as the decrease in the volume of the box determined in Equation (1) above (ΔV). As the lung volume increases, the pressure measured at the mouth decreases, as predicted by Boyle’s law:
PMi × VLi = PMf × (VLi + ΔV) (2)
where (Vli + ΔV) = Vlf
The ΔV in Equation (2) is equal to that solved for in Equation (1) and Vli is now solved for. It is the FRC, since the airway was occluded at the end of a normal tidal expiration. In current practice, the patient makes several panting inspiratory efforts against the closed airway, and all the calculations described above are made automatically by a computer receiving inputs from the pressure transducers.
Anatomic Dead Space and Alveolar Ventilation
![]() The volume of air entering and leaving the nose or mouth per minute, the minute volume, is not equal to the volume of air entering and leaving the alveoli per minute. Alveolar ventilation is less than the minute volume because the last part of each inspiration remains in the conducting airways and does not reach the alveoli. Similarly, the last part of each expiration remains in the conducting airways and is not expelled from the body. No gas exchange occurs in the conducting airways for anatomic reasons: The walls of the conducting airways are too thick for much diffusion to take place; mixed venous blood does not come into contact with the air. The conducting airways are therefore referred to as the anatomic dead space. They correspond to the conducting zone in Figure 1–2.
The volume of air entering and leaving the nose or mouth per minute, the minute volume, is not equal to the volume of air entering and leaving the alveoli per minute. Alveolar ventilation is less than the minute volume because the last part of each inspiration remains in the conducting airways and does not reach the alveoli. Similarly, the last part of each expiration remains in the conducting airways and is not expelled from the body. No gas exchange occurs in the conducting airways for anatomic reasons: The walls of the conducting airways are too thick for much diffusion to take place; mixed venous blood does not come into contact with the air. The conducting airways are therefore referred to as the anatomic dead space. They correspond to the conducting zone in Figure 1–2.
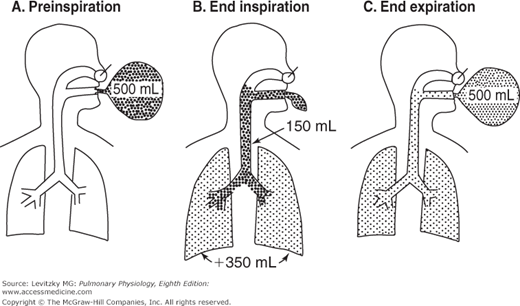
The anatomic dead space is illustrated in Figure 3–7. A subject breathes in from a balloon filled with 500 mL of a test gas such as helium (mixed with oxygen) that is not taken up by or liberated from the pulmonary capillary blood. Initially (Figure 3–7A), there is no test gas in the subject’s airways or lungs. The subject then (Figure 3–7B) breathes in all 500 mL of the gas. However, not all the gas reaches the alveoli. The final portion of the inspired gas remains in the conducting airways, completely filling them. The volume of the test gas reaching the alveoli is equal to the volume breathed in from the balloon minus the volume of the anatomic dead space, in this case 500 mL – 150 mL, or 350 mL. The 350 mL of test gas mixes with the air already in the alveoli and is diluted. During expiration (Figure 3–7C) the first gas breathed back into the balloon is the undiluted test gas that remained in the anatomic dead space. Following the undiluted test gas is part of the gas that reached the alveoli and was diluted by the alveolar air. The last 150 mL of alveolar gas breathed out remains in the anatomic dead space. The concentration of test gas collected in the balloon after expiration is lower than it was before the breath but higher than the concentration left in the alveoli and conducting airways because it is composed of pure test gas from the anatomic dead space and diluted test gas from the alveoli.
Figure 3–7.
Illustration of the anatomic dead space. A: The subject inspires 500 mL from a balloon filled with a high concentration of a test gas (denoted by the dots). B: At the end of the inspiration, only 350 mL of the test gas has reached the alveoli. This 350 mL is added to the 2 to 3 L of alveolar gas already in the lungs at the functional residual capacity, and so its concentration is diluted. The other 150 mL of test gas remains virtually unchanged in the subject’s anatomic dead space. C: At end expiration, diluted test gas remains virtually unchanged in the subject’s anatomic dead space, and it remains equally concentrated in alveolar air and in the anatomic dead space. The test gas in the balloon is a mixture of undiluted gas from the dead space and diluted alveolar gas.
Therefore, for any respiratory cycle, not all the VT reaches the alveoli because the last part of each inspiration and each expiration remains in the dead space. The relationship among the VT breathed in and out through the nose or mouth, the dead space volume (Vd), and the volume of gas entering and leaving the alveoli per breath (Va) is:
Thus, if a person with an anatomic dead space of 150 mL has a VT of 500 mL per breath, then only 350 mL of gas enters and leaves the alveoli per breath.
The alveolar ventilation (per minute) can be determined by multiplying both sides of the above equation by the breathing frequency (n) in breaths per minute:
The alveolar ventilation 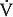 in liters per minute is equal to the minute volume (
in liters per minute is equal to the minute volume (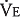 ) minus the volume wasted ventilating the dead space per minute (
) minus the volume wasted ventilating the dead space per minute (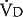 ):
):
The dot over the letter V indicates per minute. The symbol 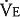 is used because expired gas is usually collected. There is a difference between the volume of gas inspired and the volume of gas expired because as air is inspired, it is heated to body temperature and humidified and also because normally less carbon dioxide is produced than oxygen is consumed. (See the alveolar air equation on pages below.)
is used because expired gas is usually collected. There is a difference between the volume of gas inspired and the volume of gas expired because as air is inspired, it is heated to body temperature and humidified and also because normally less carbon dioxide is produced than oxygen is consumed. (See the alveolar air equation on pages below.)
Measurement of Alveolar Ventilation
Alveolar ventilation cannot be measured directly but must be determined from the VT, the breathing frequency, and the dead-space ventilation, as noted in the previous section.
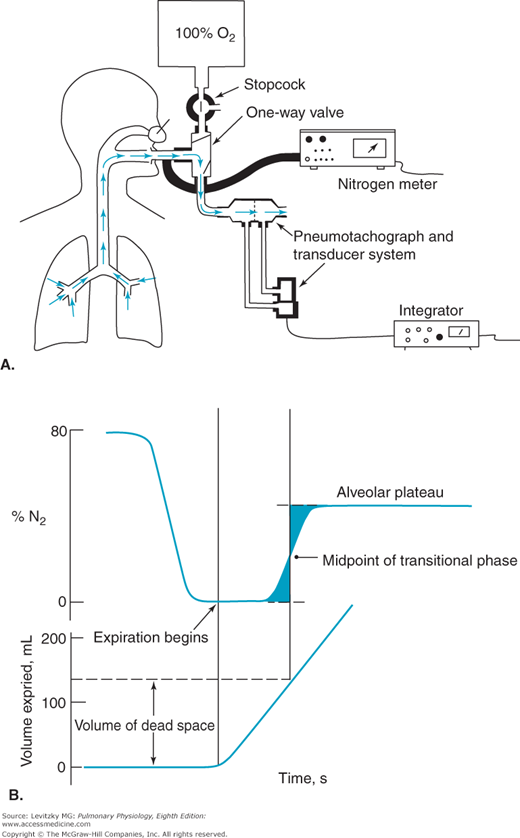
For a normal, healthy subject, the anatomic dead space can be estimated by referring to a table of standard values matched to sex, age, height, and weight or body surface area. A reasonable estimate of anatomic dead space is 1 mL of dead space per pound of ideal body weight. The anatomic dead space can be determined by using Fowler’s method. This method uses a nitrogen meter to analyze the expired nitrogen concentration after a single inspiration of 100% oxygen. The expired gas volume is measured simultaneously. Fowler’s method is summarized in Figure 3–8.
Figure 3–8.
Fowler’s method for the determination of anatomic dead space. A: The subject takes a single breath of 100% oxygen, holds his or her breath for a second, and then exhales. Nitrogen concentration is monitored along with the volume of gas expired, in this case by integrating with time the airflow (L/s) determined by a pneumotachograph-differential air pressure transducer system. B: The volume of gas expired between the beginning of the exhalation and the midpoint of the rising phase of the expired nitrogen concentration trace is the anatomic dead space. (The midpoint is determined such that the 2 shaded areas are equal.)
The subject breathes in a single breath of 100% oxygen through a 1-way valve, holds it in for a second, and then exhales through the 1-way valve. Nitrogen concentration at the mouth and the volume expired are monitored simultaneously.
Initially, the nitrogen concentration at the mouth is 80%, the same as that of the ambient atmosphere. As the stopcock is turned and the subject begins to inspire 100% oxygen, the nitrogen concentration at the mouth falls to zero. The subject holds his or her breath for a second or so and then exhales through the valve into a spirometer or pneumotachograph. The first part of the expired gas registers 0% nitrogen because it is undiluted 100% oxygen from the anatomic dead space. In the transitional period that follows, the expired gas registers a slowly rising nitrogen concentration. During this time, the expired gas is a mixture of dead-space gas and alveolar gas because of a gradual transition between the conducting pathways and the respiratory bronchioles, as was seen in Figure 1–2. The final portion of expired gas comes solely from the alveoli and is called the alveolar plateau. Its nitrogen concentration is less than 80% because some of the inspired breath of 100% oxygen reached the alveoli and diluted the alveolar nitrogen concentration, as shown in Figure 3–7. The volume of the anatomic dead space is the volume expired between the beginning of the expiration and the midpoint of the transitional phase, as shown in Figure 3–8. Fowler’s method is rarely used clinically.