83
CHAPTER OUTLINE
■ APPROACH TO THE PREGNANT WOMAN
■ NEONATAL ABSTINENCE SYNDROMES
■ TOBACCO
■ OPIOIDS
■ CANNABIS
■ MATERNAL ADDICTION TREATMENT
APPROACH TO THE PREGNANT WOMAN
Substance use during pregnancy has potentially significant effects on the fetus, neonate, and developing child, as well as on the mother. For these reasons, all health care providers should be able to recognize perinatal substance use and addiction, address this medical problem, and thus reduce potential complications for the mother and her child. The prevalence of substance use during pregnancy is substantial. Women addicted to or abusing tobacco, alcohol, prescription medications, and illicit substances may have irregular menstrual cycles, yet still have the ability to conceive. Several months may lapse before a woman realizes that she is pregnant (1). Perinatal substance use and addiction affect women of all races, ethnicities, and socioeconomic levels (2,3). Alcohol is estimated to be consumed by 9.4% of pregnant women, while 2.6% reported binge drinking, and 0.4% reported heavy drinking (3). The prevalence of illicit drug use varies by drug type during pregnancy, with 9% to 15% reporting marijuana use and around 2% using opioids (4) and 1% to 10% using cocaine (5,6). Pregnancy motivates some, but not all, women to quit using tobacco, alcohol, nonmedical use of prescription medications, and/or illicit drugs (5). Others may have difficulty stopping due to the severity of addiction or fear of withdrawal.
The clinician’s role is to begin to support substance-using pregnant women (SUPW) in changing their behavior to stop or substantially reduce substance use and to assist with addiction treatment referral. Whether or not she continues to use, every SUPW should be encouraged to engage in prenatal care.
Pregnant women who use psychoactive substances are much more stigmatized than nonpregnant women, so they may deny their drug use, its harmful effects, and the need to seek help (7). Clinicians who provide health care to SUPW should be sensitive to the cultural background, family context, and the patients’ feelings. Thus, clinicians need to provide care in a supportive and nonjudgmental manner. Clinicians should be sensitive and explicitly discuss with their patients the need for and limits around confidentiality regarding substance use diagnoses, because of stigma and potential legal ramifications. In many cases, a woman may have used alcohol or drugs during a previous pregnancy, experiencing the negative consequences of stigmatization and perhaps loss of custody of her other children. Substance-using and addicted pregnant women may be wary of health care providers owing to previous experiences. The availability of a physician within a prenatal clinic setting able to make the determination of at-risk use, abuse, or addiction and with skills in facilitating a referral to treatment is advantageous. This process will increase access for pregnant women to these services. Such a strategy helps to decrease the stigma attached to specialized addiction treatment services.
Encouragement and consistency in approach, expectations, and messages to SUPW are important and will maximize engagement and retention in treatment (8). Effective treatment for these patients and their newborns requires collaboration among multiple providers and agencies. It is essential that there be clear and direct communication among the mother’s addiction treatment physician, obstetricians, pediatricians, neonatologists, primary care physicians, nurses, anesthesiologists, psychiatrists, psychologists, social workers, and legal agencies.
Screening
Estimates vary, but 30% to 50% of women in the general population have unintended pregnancies. For opioid-dependent pregnant women, the rate is estimated to be 86% (9). Prevalence estimates for pregnancies exposed to other psychoactive substances are less known, but one could expect that the overwhelming majority of SUPWs did not intend to become pregnant. Thus, comprehensive care for women who may become pregnant and are using substances should include reproductive health education and access to methods to give women control over their reproductive options.
SUPW may not fit the usual stereotype of a person with addiction, making early identification difficult. Although a number of questionnaires have been validated to detect alcohol use, few instruments have been validated for detection of illicit drug use—including prescription medications— during pregnancy. Screening women in a prenatal clinic with specific questions about tobacco, alcohol, and drug use hold the promise of identifying, intervening, and reducing substance use during pregnancy (10). Asking directly about current substance use can identify the risk of use and of substance use disorders to inform and educate women planning conception or who are currently pregnant. The combination of screening questions and urine toxicology has been shown to be more effective for detection of perinatal addiction than use of either one alone (11).
Information from a thorough history (including medical problems and social stressors) and a physical examination can provide clues to use of specific substances during pregnancy. Certain facets of a patient’s history, such as a family history of substance use disorders, may alert the clinician to the possibility of substance use during pregnancy. Children of parents with an alcohol use disorder have a three- to fourfold increase in risk of developing alcohol problems themselves (12). Frequent encounters with law enforcement agencies may also indicate active drug use or a drug use disorder (13). Women are often introduced to and supplied with drugs by a male partner; therefore, substance use or addiction by a current significant other should increase the index of concern (14). Given that approximately 90% of drug treatment-enrolled pregnant women smoke cigarettes, use of nicotine and/or marijuana may be an important indicator of other addictions (15). If any of these risk factors are present, there is increased likelihood of perinatal use and/or a substance use disorder, and the expectant mother should be asked about the spectrum of substance use.
Up to 8% of the general population of pregnant women are victims of physical abuse (16), but 34% of SUPW report physical abuse (17). This rate is similar to rates of victimization among nonpregnant substance users, so pregnancy is not a protective factor against domestic violence (18).
Physical, sexual, and verbal abuse by a partner is more common among women with alcohol use disorders (19), so eliciting a thorough social history adds information about consequences of addiction. These high rates of domestic violence among SUPW should prompt clinicians to ask all pregnant women about the possibility of physical or sexual abuse, especially when substance use is also identified in the woman or her partner.
Cognizance and discussion of risks and/or indications of use or substance use disorders with women can help enhance honest communication about substance use throughout pregnancy. Once identified, options for treatment of acute withdrawal syndromes or other pharmacotherapy and behavioral treatments can be offered to SUPW, if applicable.
Routine office visits for prenatal care provide an opportunity to screen for depression and other mental health issues, as dual diagnosis (coexisting substance use disorder and other psychiatric diagnosis) is not uncommon in this population. Appropriate obstetric care also includes evaluation for sexually transmitted infections with treatment of the woman and ideally treatment of her partner. Office visits allow ongoing evaluation of the woman’s psychosocial support system and may include referral to appropriate community services, if not available in the prenatal clinic setting.
Prenatal education about labor, delivery, and care of the newborn may prevent misunderstandings. Discussions with the SUPW should include details of the birth plan, including the treatment of her pain during labor and postpartum, education about the potential need for treatment of neonatal withdrawal due to maternal medications, and appropriate contraceptive methods postpartum. When possible, involvement of the patient’s significant other and support system, with maternal consent, is helpful.
Laboratory Testing
For the SUPW in treatment, and for any pregnant women with evidence of substance with use or a substance use disorder identified at the time of labor and delivery, the physical examination, social and legal history, and a maternal history of addiction, including previous treatment episodes and substance-related obstetrical or medical problems, may indicate the need for laboratory screening for substances in mother and newborn. Infant urine, meconium, and cord blood as well as maternal urine may be tested for use of legal and illegal drugs. As with any medical diagnostic evaluation, laboratory evaluation should be accompanied by a review of maternal records, maternal medical and psychiatric history, and, for those SUPW identified at the time of the birth, a complete evaluation for substance use, and substance use disorders during pregnancy (8). In addition to helping in a diagnostic evaluation of the newly identified SUPW, negative results are helpful in validating a mother’s history of engagement in treatment, recovery, and abstinence.
Initially, immunologic assays are used to screen urine, blood, and meconium for psychoactive substances.
Positive results should be confirmed with gas chromatography/mass spectroscopy since cross-reacting chemicals may be read initially as a “positive” for a drug. Substances routinely tested vary by laboratory, so the clinician should request assays keeping in mind medications prescribed or misused, drugs commonly used in the community, and relevant maternal addiction history. For example, an assay for opiates will identify heroin, morphine, hydrocodone, hydromorphone, and codeine. If the patient is abusing a prescription pain medication such as methadone, buprenorphine, oxycodone, and other synthetic and semisynthetic opioids, they will not be detected.
The advantage of testing urine is ease of collection; however, results only reflect use and fetal exposure shortly before delivery due to the short window of detection for most substances in urine. Meconium, a dark-green odorless substance produced by the fetal gastrointestinal tract beginning at 13 to 14 weeks of gestation, may also be tested for medications, drugs of abuse, and alcohol. Meconium is passed by the newborn in bowel movements most often shortly after birth. The advantage of testing meconium is information about a long window of prenatal exposure, beginning in the second trimester (20). Unfortunately, meconium may be passed in utero, thus not available at birth, or may take days to collect. These difficulties and the lag time in receiving meconium results make collection of infant urine easier and more feasible.
Meconium and urine collected from 423 mother–infant pairs and analyzed for the presence of cannabinoids, codeine, morphine, or methadone provided no advantage over analysis of maternal urine and first infant-voided urine. Meconium was slightly more effective in testing for cocaine (21). Testing of cord blood is also feasible, providing data about exposure around the time of birth. However, availability varies, and cord blood results are probably not superior to meconium and urine. In one small study, infant cord blood results for drugs of abuse were concordant with meconium more than 90% of the time for amphetamines, opiates, cocaine, and cannabinoids (22). Finally, the fetus exposed to illicit drugs is more likely to be exposed to alcohol in utero. Significant elevation of four fatty acid ethyl esters in meconium and hair is a marker for in utero alcohol exposure although not routinely available (23,24). Ethyl glucuronide (EtG) and ethyl sulfate (EtS), metabolites of alcohol, may be present in the maternal urine for 3 to 4 days after imbibing alcohol, and if drinking occurs shortly before birth, they may be identified in the newborn (25). As EtG and EtS have primarily been used in forensic settings, the utility in monitoring abstinence in the clinical setting is under investigation. Concentrations in the urine may be affected by bacteria in the urine (EtG) and may be the result of incidental exposure (chronic use of food products, hygiene products, mouthwash, or over-the-counter medications containing ethanol), affecting clinical utility (26).
Confirmed or suspected history of addiction in the mother should lead to infant and maternal screening for hepatitis B or C, human immunodeficiency virus (HIV), or other sexually transmitted diseases. Infants born to SUPW are at higher risk for these infections because of the association of drug use with high-risk sexual behaviors and intra-nasal and intravenous routes of administration. Screening facilitates early treatment and may help prevent further transmission or other complications of infection.
Teratogenicity
A teratogen is a substance that may produce an alteration in the offspring’s physical structures and/or behavior when used during gestation. The time of exposure and amount of chemical will affect whether congenital malformations or neurobehavioral problems that persist into later lifetime will occur. In many cases, a “subthreshold exposure” will not lead to malformations, whereas in others, a small dose during critical embryogenesis can lead to significant teratogenicity (27). In the human, it is difficult to attribute causation to exposure when there are confounding variables such as malnutrition, severe stress, and concurrent use of other substances. Alcohol and tobacco, alone and in combination with other substances, are known to have the most potential to cause teratogenicity in the human, but any psychoactive substance use in pregnancy, whether illicit or medicinal, always involves some degree of risk of some form of teratogenicity to the developing embryo.
It is not necessary for a pregnant woman to meet diagnostic criteria for a substance use disorder for disruption of fetal growth and development to occur. Exposure in the first trimester can result in significant problems, including pregnancy loss. The majority of development and organogenesis occurs in the first 12 weeks of pregnancy; however, exposure can cause problems during any trimester of pregnancy. Even when maternal history of substance used, amount and mode of use (injection vs. oral), and the timing of use are known, there are multiple factors that affect determination of fetal substance exposure. Substances are often used in combination, confounding the effects of exposure (28). Additionally, recall bias may impact the validity of a retrospective maternal history. Finally, SUPW may feel guilty, ashamed, and overwhelmed when faced with problems in the newborn that are secondary to their addiction and may either under- or overreport their use of substances.
NEONATAL ABSTINENCE SYNDROMES
Polysubstance use is the norm rather than the exception in substance-exposed pregnancies; thus, it is often difficult to determine specific effects of individual drug exposure. In the newborn, signs of intoxication as well as withdrawal from illicit and legal drugs are all characterized by autonomic instability, central nervous system (CNS) irritability, and feeding difficulties. The substance-exposed newborn may have poor weight gain, instability in heart rate, respiratory rate, and temperature, as well as hyperactivity, irritability, hypertonia or hypotonia, difficulty sucking or excessive sucking, sleep disturbance, and high-pitched cries (29).
The duration and severity of intoxication and the onset of withdrawal syndrome will depend upon the time of the last drug exposure, the combination of substances, and the metabolism and excretion of the drug. For example, an infant exposed to cocaine will be irritable, have sleep/wake cycle disruption, and feed poorly, but if the infant has also been alcohol exposed, symptoms may be more severe, as alcohol and cocaine form cocaethylene, a potent stimulant (30). Timing of withdrawal also varies. In the case of the infant exposed to heroin, withdrawal signs will generally emerge in the first 24 hours of life for the newborn if use occurred shortly before birth. In contrast, if methadone is prescribed or abused, signs of withdrawal usually present later than with heroin, after 48 to 72 hours postbirth (31).
When the diagnosis of substance use or maternal substance use disorder is under consideration, there is known use of tobacco, or a urine drug screen has been positive for substances, newborns should have regular assessment for withdrawal or intoxication beginning at birth or as soon as possible. Additionally, an infant born to a mother currently prescribed opioid medication for treatment of her addiction should be monitored closely. Initial treatment of the neonate experiencing neonatal abstinence syndrome (NAS) should be primarily supportive, as pharmacotherapy may prolong hospitalization and subject the neonate to exposure to medications that may not be indicated (32).
The substance-exposed neonate is easily overstimulated, so ambient light exposure and noise should be minimized. Quieting the infant with swaddling, frequent small feedings, and intravenous replacement of fluids and electrolytes may be required. Indications for pharmacotherapy include seizures; poor feeding, diarrhea, or vomiting resulting in dehydration or excessive weight loss; inability to sleep; or significant autonomic instability with bradycardia or tachycardia, apnea or tachypnea, or temperature instability not due to infection. Pharmacotherapy should be considered if the infant is too ill to assess possible withdrawal signs, if comorbid medical problems dictate that the infant will not tolerate NAS, or if the infant is not eating well and not thriving as expected. Vomiting and diarrhea associated with dehydration and poor weight gain, in the absence of other diagnoses, are relative indications for treatment, even without high total withdrawal scores (32).
Clinical signs and symptoms should not be attributed solely to drug withdrawal or intoxication without appropriate assessment and diagnostic tests to rule out other causes. The differential diagnosis for NAS includes sepsis, hypoglycemia, perinatal anoxia, intracranial bleed, and hyperthyroidism (33). Consultation with a neonatologist may be indicated in cases where presentation is not straightforward, the course is difficult, or the infant is not responding to pharmacotherapy and supportive measures.
Neonates with intrauterine drug exposure should be followed up in the hospital for at least 72 to 96 hours after birth to monitor for signs of a neonatal intoxication or a withdrawal syndrome. If an infant is discharged prior to this time, the mother and her support network should be well informed about signs of NAS. She will need clear instructions about how to reach a physician to assess the newborn should signs emerge. If more than 7 days has elapsed between the last maternal use and delivery, the incidence of NAS is low (34).
TOBACCO
Forty percent of women who smoke and become pregnant quit during pregnancy (35). All pregnant women should be asked directly about smoking and any quit attempts. The American College of Obstetricians and Gynecologists (ACOG) recommends clinicians strongly advise all pregnant tobacco users to quit (36). Nicotine replacement has not been found to be successful with pregnant women, and the U.S. Preventive Services Task Force has indicated that there is insufficient information regarding its safety and efficacy (37). Varenicline and bupropion are alternative pharmacotherapies for smoking cessation. However, varenicline has no known safety data for use in pregnancy, and limited research suggests that bupropion has no known adverse fetal or maternal effects (38). Both medications now contain FDA–mandated product labels warning of risk of psychiatric symptoms and suicide linked to their use. Moreover, both medications can be found in breast milk. Thus, it is suggested that health care providers focus on brief office-based interventions coupled with referral to smoking cessation programs specifically developed for pregnant women. Finally, SUPW should be reminded that quitting at any time during their pregnancy is advantageous to themselves, their fetus, and their other children (39).
Cigarette use exposes the fetus to carbon monoxide, nicotine, and tar, which contains multiple chemicals including cyanide and lead. Disruption of growth has been demonstrated in the animal model and is due to intrauterine hypoxia, a result of carbon monoxide and other metabolites causing reduced uterine blood flow (40). Nicotine is quickly absorbed, crosses the placenta, and is active in the developing CNS, causing developmental neurologic problems (41). An inverse relationship exists between birth weight and the number of cigarettes smoked per day. Neonates born to mothers who smoked during pregnancy weigh an average of 200 g (range, 100 to 400 g) less and have lower birth lengths than neonates born to mothers who did not smoke during pregnancy (42). Fortunately, a period of accelerated growth occurs during the first year of life, and generally, no differences in body weight or length are observed among these infants at 1 year of age.
Because nicotine is a stimulant, infants born to women who smoke or chew tobacco may also be irritable and difficult to calm. When assessed with the Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS), infants exposed to tobacco were more excitable and hypertonic with indications of disturbance in the CNS, gastrointestinal system, and visual response (43). Some investigators have proposed development of a scoring system to describe a nicotine withdrawal syndrome (44).
Neurobehavioral abnormalities have been identified in the newborn period in infants exposed to tobacco in utero (43). Multiple studies have examined the causal link between sudden infant death syndrome (SIDS) and smoking. Given the confounding effect of postnatal exposure to cigarette smoking, an analysis of more than 60 studies concluded that nearly one-third of SIDS deaths may be prevented with cessation of smoking in pregnancy (45).
ALCOHOL AND SEDATIVES
More than 60% of women who use alcohol and become pregnant quit during pregnancy (36). The U.S. Surgeon General’s office advises that women who are pregnant or considering pregnancy should not drink alcohol (4). The majority of women who abuse sedatives will take multiple drugs, including benzodiazepines, barbiturates, and other sleeping pills, as well as drink alcohol. The smell of alcohol on the breath indicates recent ingestion but not necessarily acute intoxication. Palpation of the abdomen may reveal an enlarged or shrunken liver due to alcoholic hepatitis. Even a minimal neurologic evaluation can reveal altered mental status due to intoxication or acute alcohol withdrawal. Hyperreflexia and tremulousness may also prompt consideration of acute alcohol withdrawal.
Progression to severe withdrawal from alcohol or sedatives carries a significant mortality risk, so early recognition and treatments are essential. However, the normal physiologic changes that accompany pregnancy can make it difficult to recognize early withdrawal. Table 83-1 displays similarities and differences between sedative withdrawal syndrome and pregnancy. Treatment for acute withdrawal from sedatives (including alcohol) in SUPW should be accomplished in an inpatient setting that allows for medical supervision in collaboration with an obstetrician. Uncontrolled withdrawal symptoms may be life threatening to both the mother and fetus. Benzodiazepines and barbiturates can adversely affect the fetus when given during pregnancy, so this should be taken into account when beginning treatment for acute withdrawal symptoms. However, the risk to both mother and fetus from untreated sedative withdrawal is usually greater than the potential risk to the fetus from exposure to these medications in a controlled setting.
TABLE 83-1 PREGNANCY AND SEDATIVE–HYPNOTIC WITHDRAWAL
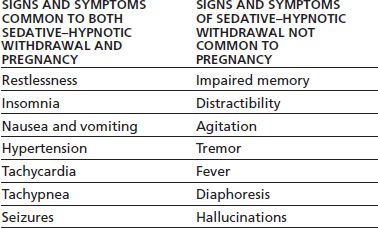
In the pregnant woman, when alcohol is consumed, it is absorbed into the maternal bloodstream, quickly crosses the placenta, and enters fetal circulation. Alcohol is found in significant levels in the amniotic fluid even after a single moderate dose. As fetal hepatic circulation does not metabolize alcohol as efficiently as in the adult, alcohol is not eliminated from the amniotic fluid as rapidly as it is from maternal circulation (27).
Fetal Alcohol Syndrome
Over the last 40 years, multiple diagnostic criteria and schemata have been proposed to describe the spectrum of structural anomalies and neurocognitive disabilities associated with alcohol exposure in pregnancy. Although diagnostic criteria and categorization remain under discussion, fetal alcohol syndrome (FAS) is still the currently accepted diagnosis that describes affected individuals. The diagnostic use of fetal alcohol effects has fallen out of favor because of the inherent ambiguity of this diagnostic classification (46). Fetal alcohol spectrum disorders (FASD) is an umbrella term that describes the range of effects that may manifest in an individual whose mother drank alcohol during pregnancy. These effects may include physical, mental, behavioral, and/or learning disabilities with possible lifelong implications (46–48). The term FASD is not intended for use as a clinical diagnosis.
Addiction treatment is an opportunity to intervene with the current pregnancy and prevent further alcohol-affected pregnancies. A woman may disclose significant history of alcohol use during prior pregnancies, and the addiction clinician may be the first professional with whom she shares concern about her other children’s development. The physician can suggest resources for a diagnostic evaluation (49).
The classic phenotype and diagnosis of FAS includes the following:
■ Evidence of growth retardation (prenatal and/or postnatal): height and/or weight equal to or less than the 10th percentile, corrected for racial norms
■ Evidence of deficient brain growth and/or abnormal morphogenesis, including one or more of the following: structural brain anomalies or head circumference equal to or less than the 10th percentile (microcephaly)
■ Evidence of a characteristic pattern of minor facial anomalies, including two or more of the following: short palpebral fissures (equal to or less than the 10th percentile), thin vermillion border of the upper lip, and smooth philtrum
Of note, diagnostic criteria for FAS have been clarified by publication of a nomogram for determining palpebral fissure length centiles. Additionally, the 5-point pictorial score for assessing philtrum smoothness and thickness along the vermillion border provides further diagnostic guidance (47).
Fetal Alcohol Spectrum Disorders
The umbrella term fetal alcohol spectrum disorder expands the classification of prenatally alcohol-exposed individuals to include the following:
■ FAS with and without confirmed maternal alcohol exposure
■ Partial FAS: This diagnostic classification allows for a known or unknown maternal alcohol exposure history. It includes evidence of the characteristic pattern of minor facial anomalies and evidence of either prenatal and/or postnatal growth retardation or structural brain abnormalities or microcephaly.
■ Alcohol-related birth defects (ARBD): Alcohol exposure in pregnancy can cause significant structural defects in multiple organ systems known as ARBD. This includes anomalies in multiple organ systems such as cardiac (atrial septal defect, ventricular septal defect, conotruncal anomalies), skeletal (radioulnar synostosis and vertebral defects), renal (aplastic/hypoplastic/dysplastic kidneys), eyes (strabismus, ptosis, vascular and nerve anomalies), and ears (conductive and sensorineural hearing defects) and may include minor anomalies of the hands, ears, and chest wall with pectus carinatum/excavatum (47).
■ Alcohol-related neurodevelopmental disorder (ARND): In addition to microcephaly and structural abnormalities of the CNS, alcohol exposure may lead to ARND. When the diagnosis of ARND is made, there is evidence of a complex pattern of behavior or cognitive abnormalities inconsistent with developmental level. Additionally, the deficits cannot be explained by genetic predisposition, family background, or environment alone in the exposed child. The child or adult with ARND has marked impairment of complex developmental tasks, higher-level receptive and expressive language deficits, and disordered behavior (50).
For further clarification of the diagnosis and referral of alcohol-exposed individuals, current guidelines are available from the Centers for Disease Control and Prevention (50). The reader is also referred to the comprehensive review by Hoyme et al. (47).
The prevalence of FASD and FAS varies across populations, but affected adults and children are found in all races and ethnic groups. Poverty often leads to higher rates of alcohol use disorders, fetal exposure, and FAS as well as FASD (51). In the United States, it is estimated that between ½ and 2 per 1,000 live births will meet diagnostic criteria for FAS (51). FASD is more common, encompassing a wider spectrum of effects of exposure, and up to 1% of live births may be affected (52).
After identification of a potential case, the clinician should refer the family for a multidisciplinary evaluation of the affected child, as the differential diagnosis for the clinical features of FAS and FASD includes other genetic syndromes and fetal exposures to other teratogenic substances. Such an evaluation should include a physician skilled in the diagnosis of developmental disabilities, a psychologist with the ability to perform neuropsychologic evaluation, educational specialists, and physical, occupational, and speech and language professionals (47). Addiction clinicians may refer families seeking information about FAS and FASD to the National Organization of Fetal Alcohol Syndrome (NOFAS; www.nofas.org).
Barbiturates such as phenobarbital have been evaluated as part of a syndrome of anticonvulsant embryopathy described in infants exposed to anticonvulsant drugs in utero (53). Features include increased major malformations, growth retardation, and hypoplasia of the midface and fingers. The Antiepileptic Drug Pregnancy Registry (AEDPR) enrolled 77 pregnancies to determine the risk of embryopathy with exposure, including phenobarbital as well as other mood stabilizing medications. The AEDPR found the relative risk of major congenital malformations in anticonvulsant-exposed offspring was 4.2 and there was no difference in exposure to phenobarbital when compared to the other medications (54). The maternal use of barbiturates near term, particularly at chronic, high doses, can result in respiratory depression and a barbiturate withdrawal syndrome in the neonate (55).
The use of benzodiazepines during pregnancy has been associated with various degrees of teratogenic effects, particularly cleft lip and palate. However, data are conflicting, and heavy benzodiazepine use often is associated with exposure to alcohol and other substances (56). Overall, the use of benzodiazepines during pregnancy appears to have a low teratogenic risk (27). Maternal misuse of benzodiazepines near term has resulted in poor muscle tone and respiratory depression in the neonate (57).
In newborns, sedative withdrawal is less common than opioid withdrawal syndrome. The newborn exposed to a long-acting benzodiazepine such as diazepam will display withdrawal signs much later than one exposed to short- acting sedatives like alprazolam or alcohol. Exposure to benzodiazepines and/or barbiturates in addition to opioids may cause even later onset of opioid withdrawal signs (58).
Symptoms of neonatal sedative withdrawal syndrome, including alcohol, are similar to opioid withdrawal syndrome. In most cases, exposure to alcohol occurs in tandem with other substances, with tobacco the most commonly co-occurring substance. The development of a sedative scoring scale has been difficult because of the predominance of polysubstance exposures, and, therefore, there is no specific sedative scoring scale. Seizures are more frequent with alcohol withdrawal than opioid withdrawal syndrome, though, given the short half-life of alcohol, even in the newborn, the need for the treatment of alcohol withdrawal is rare (59). Additionally, neonatal benzodiazepine withdrawal syndrome usually resolves spontaneously and does not require specific treatment, though phenobarbital is the agent of choice for severe sedative withdrawal syndrome. Clinical trials comparing dosage, dosing intervals, and the need for serum levels of phenobarbital have not been done. Phenobarbital can be administered orally or intramuscularly at a dose of 2 to 4 mg/kg of body weight every 8 hours (60). After stabilization with reduction of neonatal withdrawal signs, the dose can be tapered by 10% to 20% per day over 5 to 10 days (32).
OPIOIDS
Use of heroin or nonmedical use of prescription opioid medications may continue into pregnancy because attempts to quit lead to obvious withdrawal symptoms, but SUPW may be reluctant to disclose information about their opioid use. Constipation from opioid use may be apparent on abdominal examination, although this is nonspecific and may be related to pregnancy. Opioids may be used by injection, especially heroin, so clues on screening that indicate injection drug use are useful. Nearly half of parenteral drug users have a history of acute hepatitis (19). Infections such as endocarditis, recurrent cellulitis, or thrombophlebitis should raise suspicion for injection drug use. Some SUPW hide needle marks by injecting under the tongue, in the axillae, under the breasts, into the legs, between fingers or toes, and under the nails. Track marks are wormlike scars from repeated injection that follow the courses of veins. Look for healed abscess scars from subcutaneous injection.
Maternal Pharmacotherapy
Opioid withdrawal syndrome during pregnancy can lead to fetal distress and premature labor owing to increased oxygen consumption by both mother and fetus. Even minimal symptoms in the mother may indicate fetal distress, as the fetus may be more susceptible to withdrawal symptoms than the mother. Methadone is frequently used to treat acute withdrawal symptoms from illicit opioids. Methadone may be used by a physician for temporary maintenance when an addicted patient is admitted to a hospital for an illness other than opioid addiction. This includes admission for evaluation for preterm labor, which may be induced by acute withdrawal. Naloxone should not be given to a pregnant woman except as a last resort in life-threatening opioid overdose, as withdrawal precipitated by an opioid antagonist can result in spontaneous abortion, premature labor, or stillbirth.
Medical withdrawal of the pregnant opioid-dependent woman is not recommended because of high rates of relapse to illicit prescription opioid and heroin use and the increased risk to the fetus of intrauterine death. Methadone maintenance has served as the long-time standard of treatment (61). Being on an adequate and stable methadone dose decreases fluctuations in maternal opioid level, which is thought to reduce stress on the fetus related to rapid cycles of intoxication and withdrawal. Fluctuations between opioid intoxication and withdrawal result in adverse fetal effects, such as premature labor and spontaneous abortion. Illicitly bought heroin is likely adulterated with other compounds that may be harmful to the fetus, and access to illicit prescription opioids may be unpredictable, so elimination of any opioid use with adequate doses of methadone prevents harm to the fetus from exposure to these other compounds. Engagement in medication-assisted treatment improves maternal health and nutrition, reduces obstetric complications, and improves the health of the infant at delivery. Other advantages of methadone maintenance over illicit opioid use are reduction of criminal activity and decreased disruption of the maternal– child dyad (62). Methadone maintenance enhances the ability of SUPW to participate in prenatal care and addiction treatment, thus giving the woman and her family the opportunity to adequately prepare for the arrival of the infant.
Opioid-dependent pregnant women should be referred to a local methadone maintenance program, if available. Most programs assign high priority to pregnant women, so the patient may be able to enter treatment sooner than if she were not pregnant. Opioid-dependent pregnant women on methadone maintenance are monitored regularly throughout the pregnancy, and the dose is adjusted as necessary. Maternal methadone dose does not correlate with neonatal abstinence symptoms, so maternal benefits of methadone are not offset by harm to the newborn (63). Effective daily methadone dose in opioid-dependent pregnant women may vary (64). It is reasonable to expect the methadone dose requirement to increase during the third trimester of pregnancy. This increase is due to larger plasma volume, decreased plasma protein binding, increased tissue binding, increased methadone metabolism, and increased methadone clearance in the mother. As a result, the half-life of methadone is shortened late in pregnancy, and the woman may experience mild withdrawal symptoms unless adjustments are made to her methadone dose. Splitting the total daily methadone requirement into two doses, given in the morning and evening, is preferred (65,66), if possible. This split-dose procedure provides a more even blood concentration throughout each day. Methadone maintenance as part of comprehensive care for pregnant heroin users and illicit prescription opioid users improves maternal psychosocial function and birth outcomes (67).
Sublingual buprenorphine, a partial μ agonist and κ antagonist prescribed for the treatment of opioid addiction, like methadone, has not yet been approved for use in pregnancy by the U.S. Food and Drug Administration, but has been used successfully for opioid maintenance in pregnant women (68–70). This medication has been well tolerated by pregnant women, and newborns have had a similar incidence of NAS compared to mothers on methadone, yet appear to require less morphine to treat and shorter duration of treatment for NAS (64).
Neonatal Withdrawal
Neonatal opioid withdrawal syndrome occurs in 60% to 80% of infants with intrauterine exposure to heroin or prescription opioids, including methadone and buprenorphine (71). The incidence of the syndrome is best described in those cases where SUPW are prescribed an opioid while in treatment. In these cases, historical and laboratory information for most of the pregnancy is available and may assist in treatment of the newborn (72).
Assessment and pharmacologic management of opioid-exposed infants vary across nurseries (73). Perhaps the most comprehensive assessment is provided by the Maternal Opioid Treatment: Human Experimental Research (MOTHER) NAS Measure (64) (Fig. 83-1 and Table 83-2). This modified Finnegan scale with specified item by item definitions assesses signs and symptoms with weighted scores, which are evaluated at 2 hours after birth and then every 4 hours. In scoring an infant, considerations of other factors, such as comorbid exposure to tobacco or other stimulants, a noisy environment and overstimulation, or whether the infant is hungry or not, may impact upon results. Pharmacotherapy is usually initiated when the total score is 9 or greater for 2 consecutive evaluations or 13 or more on a single evaluation, with dose escalation if scores climb rather than drop; however, some nurseries will utilize a lower score to initiate pharmacotherapy in an infant with comorbid medical problems or a higher score if the infant is feeding well and thriving. After initiation of treatment, the clinician should evaluate and score the infant every 4 hours until the score remains at 8 or below for 48 hours.
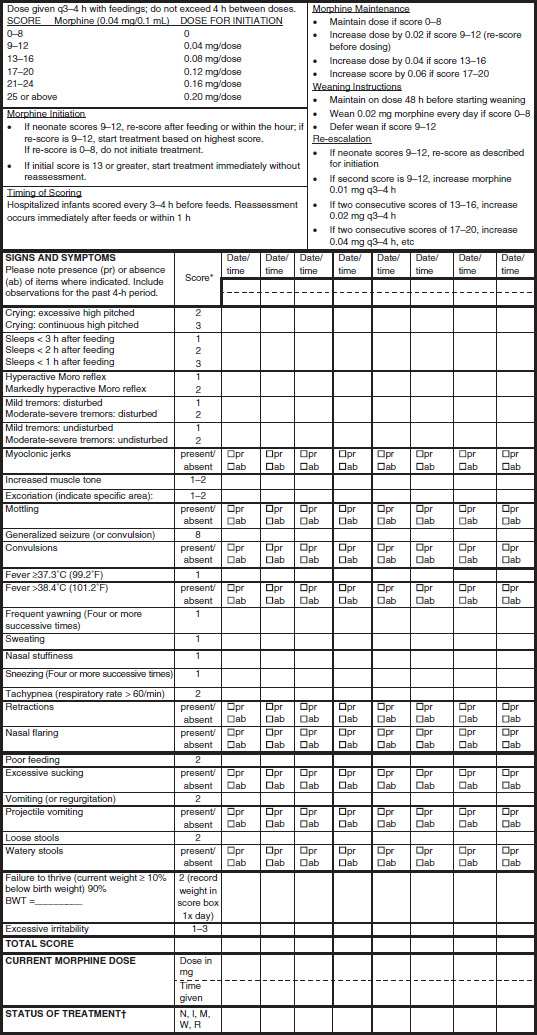
FIGURE 83-1 Maternal Opioid Treatment: Human Experimental Research (MOTHER) Neonatal Abstinence Measure. *See Table 83-2 for scoring. †Code Status of Treatment as follows: N, no treatment; I, initiation; M, maintenance; W, weaning; R, reescalation. (Reproduced from Jones HE, Kaltenbach K, Heil S, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med 2010;363:2320–2331. Copyright © 2010, Massachusetts Medical Society.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


