Adenoviruses
William S.M. Wold
Michael G. Ison
History
During attempts to establish tissue culture lines from tonsils and adenoidal tissue surgically removed from children, Rowe and colleagues613 recognized that a transmissible agent was causing degeneration of the epithelial-like cells. Thus, adenoviruses (Ads) were first cultured and reported as distinct viral agents in 1953.787
A nomenclature for Ads was adopted in 1956, and then Ads were reclassified in 1999.743 The family name is Adenoviridae, and there are four accepted genera: Mastadenovirus, from mammals; Aviadenovirus, from birds; and Atadenovirus and Siadenovirus, from a broad range of hosts.50,141,743 A fish adenovirus falls into a fifth clade.141 Human Ads are divided into seven species, A, B, C, D, E, F, and G, based on serum neutralizing and hemagglutination epitopes, genome sequence and function, oncogenic properties in newborn hamsters, and pathology in humans. These species were previously referred to as groups or subgroups.
Historically, the human Ad isolates were designated as serotypes, based on neutralization of productive infection by homologous sera.50 Recently, with the advent of high-throughput Ad genome sequencing and bioinformatics analysis, new insights have been obtained into Ad genome structure and taxonomy.141,262 Evolution of Ads seems to have been driven by not only sequence divergence but also frequent recombination between different serotypes.444,450,593,594,643,644,756,764,763 Researchers have taken the view that Ad isolates should be designated as types rather than serotypes, per definitions of the International Committee on Taxonomy of Viruses. Two similar but not exact proposals have been advanced to characterize Ads.17,642 With one proposal, for example, human Ad serotype 1 will be designated type HuAdV-1, with the “1” referring to hexon (the major capsid protein) identity. The rationale is that hexon should remain the major identifier “because it contains the major neutralizing epitope, which is targeted in molecular diagnosis”.17 With the other proposal, human Ad serotype 1 will become type HAdV-C1, with the “C” referring to species C.642 In the current article we will use serotype to indicate type because serotype has been used in nearly all the literature. According to current standard nomenclature, “H” should precede the serotype number, for example, HAdV-5 for human serotype 5. For brevity, we will use “Ad5.”
There are 57 serotypes in the seven species of human Ads: species A (Ad12, 18, 31), species B (Ad3, 7, 11, 14, 16, 34, 35, 50, 55), species C (Ad1, 2, 5, 6), species D (Ad8 to 10, 13, 15, 17, 19, 20, 22 to 30, 32, 33, 36 to 39, 42 to 49, 51, 53, 54, 56), species E (Ad4), species F (Ad40 and 41), and species G (Ad52) (Table 56.1). Species B can be further divided into species B1 (Ad3, 7, 11, 16, 21, 50) and B2 (Ad11, 14, 34, 35). Ad52 to 57 are recent isolates. Ad52 (species G) was isolated from a patient with gastroenteritis.343 Ad53357,763 and Ad54326 are in species D and associated with epidemic keratoconjunctivitis (EKC). Ad56, species D, which caused a rare neonatal fatality and keratoconjunctivitis, seems to be a complex recombinant
of Ad9, 15, 26, and 29.594 Ad55, species B, isolated from a respiratory outbreak in China, is a recombinant between Ad14 (97% of genome) and Ad11.766 An isolate from the feces of a 4-year-old patient444 was recently proposed, based on computational analysis, to be Ad57 in species C: it has a fiber similar to Ad6 but a unique hexon.765 The genome of many human Ad serotypes has been sequenced.450,643,765,766,776 Also, the genome sequence of the Ad5 reference material (ARM) was published as a reference strain for Ad5-based vectors.691 The genomes of serotypes within a species are highly related and are modestly diverged from species to species. This chapter will tend to concentrate on the relatively recent literature on Ad pathogenesis in humans. Some of the earlier literature can be found in the fourth and fifth Editions of Fields Virology.308,787
of Ad9, 15, 26, and 29.594 Ad55, species B, isolated from a respiratory outbreak in China, is a recombinant between Ad14 (97% of genome) and Ad11.766 An isolate from the feces of a 4-year-old patient444 was recently proposed, based on computational analysis, to be Ad57 in species C: it has a fiber similar to Ad6 but a unique hexon.765 The genome of many human Ad serotypes has been sequenced.450,643,765,766,776 Also, the genome sequence of the Ad5 reference material (ARM) was published as a reference strain for Ad5-based vectors.691 The genomes of serotypes within a species are highly related and are modestly diverged from species to species. This chapter will tend to concentrate on the relatively recent literature on Ad pathogenesis in humans. Some of the earlier literature can be found in the fourth and fifth Editions of Fields Virology.308,787
Table 56.1 Infections Associated with Adenovirus Subgroup and Serotype | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Infectious Agent
Propagation and Assay in Cell Culture
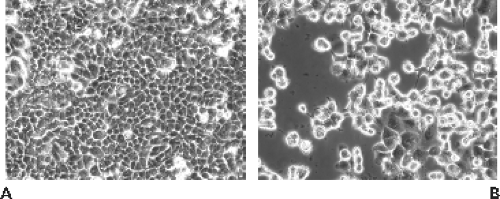
Primary human embryonic kidney (HEK) cells are probably the best host for the entire range of human Ads, but such cells are expensive and may be contaminated with adeno-associated virus. Continuous epithelial lines, such as HEp-2, HeLa, KB, HEK 293, and A549, are also highly sensitive. Ads in monolayer cell culture have a characteristic cytopathic effect (CPE). The cells round up, swell, and detach from the culture surface into grape-like clusters, and the nuclei become enlarged363 (Fig. 56.1). Eventually the cells lyse, leaving cell debris. This CPE is the result of the infection passing into the “late” stage of infection, when Ad DNA, messenger RNA (mRNA), and proteins are being made in large quantities and virions are assembling in the cell nucleus.
Biological Characteristics
Ads have a double-stranded DNA genome of ∼36,000 base pairs (bp) enclosed by a protein capsid and with no membrane (see Chapter 55). The virion binds to specific receptors on the cell surface and enters the cell by endocytosis, and the genome is transported to the cell nucleus. “Early” genes are expressed, viral DNA replicates, “late” genes are expressed, virions assemble in the cell nucleus, and the cells lyse to release progeny virus (see Chapter 55 and the legend to Fig. 56.10). There are about 20 early genes and 15 late genes. Ads increase glycolysis in continuous cell lines and thereby induce the cells to produce large quantities of acid. Rapid cytopathology can be induced within several hours of inoculating concentrated crude virus preparations and is not related to viral replication; rather, it is caused by the penton base component of the free viral penton capsomere.
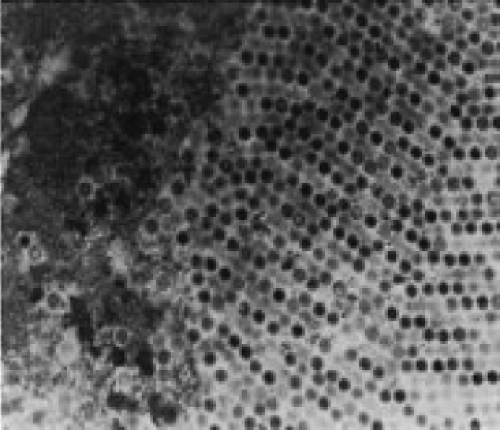
Nuclear morphologic changes in infected tissue cells can be used for diagnostic purposes. The nuclear changes include overall enlargement and intranuclear inclusions that initially are Feulgen negative and eosinophilic but become Feulgen positive and basophilic as the infection progresses.70 The Feulgen-positive inclusions contain Ads by electron microscopy (EM) (see Fig. 56.2), and it is the DNA within the viruses that
contributes to the Feulgen stain. In addition, there are paracrystalline aggregates that contain viral protein without nucleic acid.484
contributes to the Feulgen stain. In addition, there are paracrystalline aggregates that contain viral protein without nucleic acid.484
Description of Key Antigens
The clinically predominant Ad antigens are the three capsid proteins: hexon, penton base, and fiber. Most early studies indicated that the hexon and to a lesser extent the fiber proteins contain most of the epitopes recognized by neutralizing antibodies, but there are neutralizing epitopes on penton base as well (reviewed in109,218,302,448,569,591,693,786). The neutralization properties of polyclonal antibodies are often concordant with the inhibition of Ad-induced hemagglutination (HA) of selected red blood cells. However, the HA functions are the property of fiber (reviewed in521), which usually must be linked to the penton base for complete HA to occur. Because of recombination within species in clinical isolates, it is not uncommon to isolate a virus that demonstrates discordant reactions in the neutralization and HA inhibition reactions.
One recent study of naturally infected humans found that the majority of neutralizing antibodies were against the hypervariable regions of hexon, but some were also against fiber and possibly penton base.591,693 In contrast, another recent study concluded that Ad5 neutralizing antibodies to fiber are more common than to hexon in the naturally infected population, but that immunization with a replication-defective Ad5-based vector raised more neutralizing antibodies to capsid proteins other than fiber.109 Most studies in animal models infected with Ad5-based vectors have found that the predominant neutralizing antibodies are directed at hypervariable regions in hexon.404,591,810 Mechanisms by which neutralizing antibodies function include virus aggregation, virus destabilization, and blocking virus receptor interactions and integrin-mediated internalization (reviewed in670). One study with the Ad5 neutralizing hexon monoclonal antibody 9C12 reported that following infection and endosome penetration of the virus–9C12 complex, microtubule-dependent translocation to the microtubule-organizing center was inhibited.670 Another study with the same monoclonal antibody as well as polyclonal Ad5 neutralizing antibodies concluded that the cellular cytosolic protein TRIM21 binds to the antibody in the internalized antibody–virus complex and targets the virus to the proteasome for degradation.454
There are group- and type-specific epitopes on both hexon and fiber. Type-specific domains have been mapped to unique sequences in loop 1 (amino acids 281 to 292) and loop 2 (amino acids 441 to 455) of hexon by generating neutralizing antibodies to peptides from each of these regions. This epitope is referred to as the Ε determinant.448 Loops 1 and 2 had previously been shown to be on the surface of the virion by crystallography. Differences in the HA properties of rhesus and vervet erythrocytes for two important subtypes of Ad11 (Ad11p and Ad11a) have been related to nucleotide sequence differences in the shaft and knob region of fiber. The knob region of fiber, which has HA properties that are used for HI (hemagglutination inhibition) tests, includes the γ determinant.448 Ad11p and Ad11a have some differences in tissue tropism in that Ad11p can be persistent in the urinary tract and Ad11a causes acute respiratory tract infections. These properties of the whole virus may be related to the changes in fiber polypeptides that are otherwise identical for 92.3% of their amino acids.468
There are group-reactive antibodies that react with conserved domains of hexon from all human serotypes.529 These interactions were classically measured by the complement fixation (CF) test and were useful in identifying an agent as an Ad. Subsequent serologic techniques, such as immunofluorescent (IF) antibody and enzyme-linked immunosorbent assay (ELISA) determinations, were also capable of detecting group reactivity shared by most of the human Ads.
Ad-specific CD4+ T lymphocytes have been detected in peripheral blood monocytes (PBMCs) in nearly all naturally infected humans of all ages (reviewed by409).84,102,278,321,414,536,541,542,750 When PBMCs were stimulated in bulk culture (e.g., by incubation with intact Ad particles, Ad-infected cell extracts, or purified Ad proteins or with pools of peptides that span various Ad proteins), CD4+ T lymphocytes specific to hexon were identified from healthy donors.199,540,542,636,750,814 Other studies using peptides corresponding to parts of several Ad proteins identified a number of CD4+ T-cell epitopes, including the dominant human leukocyte antigen (HLA) DP4-restricted H910-924 epitope, located in the base of the hexon protein, conserved among Ad serotypes, and detectable in PBMCs from 78% of healthy adults analyzed.540,700 Many other CD4+ T-cell epitopes located in the conserved regions of Ad5 hexon and that are conserved across Ad serotypes have been identified that are recognized by PBMCs.410,411,636,750,813 In one study in which PBMCs from 44 healthy donors were analyzed, 10 CD4+ T-cell immunodominant hexon epitopes were detected in more than 50% of subjects examined.636 The HLA restriction element for some of these peptides is known.636,813 Although not as frequently found as are Ad- and hexon-specific CD4+ T cells, hexon-specific CD8+ T cells have also been detected in PBMCs from healthy donors; the cells are cross-reactive against various Ad serotypes, they secrete interferon-γ, and they have cytolytic activity in culture.199,320,321,345,410,414,669,700,813 CD4+ and CD8+ T cells specific to Ad proteins other than hexon, including penton base411 and the DNA polymerase,344,345 have also been identified.199 There is increasing evidence that cytotoxic T lymphocytes (CTLs) specific to hexon are protective in humans.410 Indeed, T-cell “lines” (mixture of CD4+ and CD8+ cells) specific to hexon (and other Ad proteins) have been shown to be effective by adoptive cell transfer in treating infection by various Ad serotypes in allogeneic hematopoietic stem cell transplant patients.198,411,413,813 The therapeutic effect is believed to be through the coordinated action of the adoptively transferred CD4+ and CD8+ T cells.813
Infection of Experimental Animals
Human Ads are mostly species specific in their replication cycle, as are most Ads of other mammals or avian species. Even nonhuman primates are poor hosts for human Ads. Human species C Ads can replicate in the lung of cotton rats,227 and the pathogenesis of Ad pneumonia has been studied in these animals.54,227,574 An ocular model of infection with either Ad5 or Ad8 has been described in cotton rats, and the clinical manifestations, including subepithelial corneal opacities, were similar to epidemic EKC.359,730 The animals shed virus, developed specific antibodies to the infecting virus, and were able to spread the infection to control cotton rats. A similar ocular model was reported in New Zealand White rabbits after topical or intrastromal inoculations of Ad5.602,729 The virus appeared to replicate, and most of the animals developed a humoral immune
response. There were findings of blepharitis, conjunctivitis, iritis, corneal edema, and subepithelial corneal infiltrates that were consistent with immune-mediated clinical disease. The Ad5 New Zealand White rabbit model has been used to evaluate the anti-Ad activity of several compounds601 including cidofovir and 2′-3′ dideoxycytidine,603 N-chlorotaurine,604 and dexamethasone povidone-iodine.123 Ad5 can also replicate in porcine tissues.723
response. There were findings of blepharitis, conjunctivitis, iritis, corneal edema, and subepithelial corneal infiltrates that were consistent with immune-mediated clinical disease. The Ad5 New Zealand White rabbit model has been used to evaluate the anti-Ad activity of several compounds601 including cidofovir and 2′-3′ dideoxycytidine,603 N-chlorotaurine,604 and dexamethasone povidone-iodine.123 Ad5 can also replicate in porcine tissues.723
A number of groups have explored the mouse as a model for pathogenesis of human Ads or as a model to evaluate oncolytic (replication-competent) Ad vectors for cancer gene therapy. The results from these studies have been mixed. Human Ads can infect cells of virtually all mammalian species including the mouse, especially if high multiplicities of infection are used (∼100 plaque forming units [pfu] per cell). The early proteins (synthesized prior to Ad DNA replication) are expressed at good levels, but most workers have found that Ad DNA does not replicate (or barely replicates), and therefore that late genes are not expressed (or are expressed at very low levels). Nevertheless, there are reports that Ad5 or Ad2 can replicate to low levels in several mouse carcinoma cell lines221,258,503,772; however, this is not a universal finding (e.g.,341). When high doses of Ad5 were inoculated intravenously into CBA mice, there appeared to be several orders of magnitude of replication in the liver.169 On the other hand, no replication was seen in the lungs of C57BL/6 mice following intranasal administration of Ad5.228 Also, little or no replication was observed in any organs following intravenous administration of an Ad5-based oncolytic vector in C57BL/6 mice801 (this vector retains all Ad genes except some in the E3 region).
Ad5 replicates modestly in cotton rat cells,726,682 canine cells,703 porcine cells,341 and Syrian hamster cells.705 In three Syrian hamster cancer cell lines, the burst size (virus yield per cell) was about 1,000, only 10-fold less than in A549 cells.705 In Syrian hamsters, about four orders of magnitude of Ad5 replication were seen in the lung following intranasal or intratracheal administration.292,487,705 Ad5 also replicates in the liver of Syrian hamsters following intravenous administration.801
Because Syrian hamster tissues are quite permissive for Ad5 and there are numerous Syrian hamster cancer cell lines, Syrian hamsters have been used as a model to investigate the toxicology and antitumor efficacy of oncolytic Ad5-based vectors.157,369,705,707 Immunocompetent and immunosuppressed (by treatment with cyclophosphamide) hamsters bearing subcutaneous tumors formed by injection with various Syrian hamster cancer cell lines were treated by intratumoral injection with a variety of oncolytic Ad vectors.63,64,66,94,95,155,156,361,384,648,677,705,706 In general, these studies show that oncolytic Ad5-based vectors suppress the growth of tumors, and that there is a rapid adaptive immune response to the vector that appears to eliminate the vector from the tumor. Syrian hamsters have also been used to study the biodistribution and toxicity of oncolytic Ad5-based vectors in advance of clinical trials.374,429,460,675,801
Immunocompetent,159,727 newborn,815 and immunosuppressed727 Syrian hamsters have been used to evaluate compounds to inhibit Ad5 replication. A mouse model was used similarly to test cidofovir against disseminated mouse Ad type 1 infection.417
Human Ads inoculated into a variety of rodent species cause tumors. Ad12, 18, and 31 are highly oncogenic in newborn Syrian hamsters, and much has been learned about the mechanism of action of the genes in the E1A and E1B regions from these viral models (see Chapter 55). The integrated Ad12 sequences in these hamster tumors are a model to understand epigenetic consequences of foreign DNA integrations.160 Ad9 causes fibroadenomas and mammary sarcomas in rats, and an early region 4 gene seems to be important in this model.405
Functions of Adenovirus E3 Proteins
The proteins coded by the Ad E3 transcription unit are believed to provide protection of infected cells from the host antiviral response (Fig. 56.3) (reviewed in57,196,259,309,430,445,465,646,785). The Ad2 or Ad5 E3-gp19K protein is a type I glycoprotein localized in the endoplasmic reticulum (ER) (reviewed in259). E3-gp19K binds to major histocompatibility complex class I (MHC-I) heavy chain in the ER, prevents transport of MHC-I to the cell surface by virtue of an ER retrieval signal on E3-gp19K, and prevents killing of Ad-infected cells by CTLs.14,82,131,582 E3-gp19K and its function are conserved among serotypes in all human Ad species212 except species A in which the E1A proteins cause down-regulation of MHC-I.340 E3-gp19K binds with higher affinity to HLA-A than to HLA-B, and it binds poorly if at all to HLA-C.212,259 E3-gp19K binds via a conserved domain (among Ad serotypes)213,641 to the outer surface of the peptide-binding groove on MHC-I molecules.259,437 E3-gp19K also binds to transporter associated with processing (TAP), prevents formation of the TAP–tapasin complex, and limits the inclusion of TAP into the peptide-loading complex of antigenic peptide, MHC-I, and chaperones.52 This property of E3-gp19K could possibly retard cell surface expression of MHC-I in individuals with HLA-B and HLA-C MHC-I molecules.259
Reduced expression of MHC-I could render Ad-infected cells susceptible to killing by natural killer (NK) cells. To potentially enhance this possibility, the Ad E1A proteins up-regulate ligands recognized by the NKG2D receptor on NK cells and sensitize the E1A-expressing cells to NK cell–mediated cell lysis.467,611 Multiple ligands for NKG2D are known, including the MHC-I chain-related A (MICA) and B (MICB) proteins. As is the case with MHC-I, E3-gp19K causes retention of MICA and MICB in the ER, prevents their transport to the cell surface, and reduces killing of E3-gp19K–expressing cells by NK cells.467,641
The Ad E3-14.7K protein inhibits tumor necrosis factor (TNF)-induced cytolysis of Ad-infected cultured cells.232,232,234,305,306,579 E3-14.7K binds to cellular IKKγ/NEMO373,426 and is reported to modulate NF-kB activity and inhibit TNF-induced apoptosis.426 E3-14.7K also is reported to bind to caspase 8.107,373 In addition, E3-14.7K inhibits internalization of TNF receptor 1 as well as formation of the death-signaling complex (DISC) that is required for TNF-induced apoptosis.625 Two studies report that E3-14.7K inhibits TNF-induced signaling through NF-kB,90,426 whereas another study did not observe such activity for E3-14.7K.625 In a recent report, E3-14.7K stably expressed in mouse cells inhibited induction by TNF of the chemokine CCL2 (monocyte chemoattractant protein-1 [MCP-1]) by preventing phosphorylation of glycogen synthase kinase-3β and recruitment of NF-kB to the CCL2 promoter.678 Some researchers107,794 but not others714 report that E3-14.7K inhibits apoptosis by Fas ligand. E3-14.7K also inhibits TNF-induced release of arachidonic acid,390,709,822 a property that might be important in reducing inflammation.
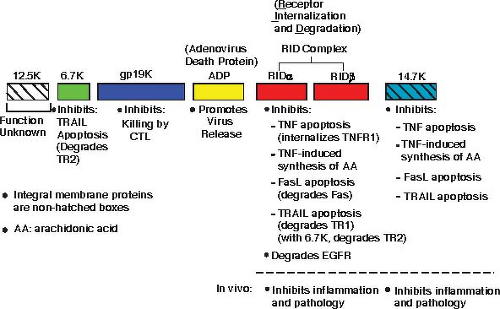 Figure 56.3. Schematic illustrating the E3 proteins and their functions. Each bar represents a protein, with the name of the protein above the bar. Colored bars without hatches are integral membrane proteins. The adenovirus death protein (ADP) was originally named the 11.6K protein.717,718 The receptor internalization and degradation (RID) protein was originally named E3-10.4K/14.5K, and RIDα and RIDβ were named E3-10.4K and E3-14.5K, respectively.714 |
Two other E3 proteins, of 10,400 daltons (10.4K) and 14,500 daltons (14.5K), which were originally named E3-10.4K716 and E3-14.5K,715 respectively, form a molecular complex originally named E3-10.4/14.5K.719 The E3-10.4K protein88 and then later the E3-10.4K/14.5K complex719 were reported to be required to clear the epidermal growth factor receptor (EGFR) from the cell surface (Fig. 56.4). The E3-10.4K and E3-14.5K proteins were later renamed receptor internalization and degradation (RID)α and RIDβ, respectively, when it was shown that these proteins also are able to cause the internalization and lysosomal degradation of not only EGFR but also cell surface Fas,180,464,662,714 TRAIL receptor 1,49,720 and TNF receptor 1.110,111 RID inhibits cytolysis induced by Fas ligand (i.e., by an agonist antibody)428,662,714 and TNF.233,390,812 RID, functioning in concert with the E3-6.7K (6,700 daltons) protein, also down-regulates TRAIL receptor 2 from the cell surface.49,427 One laboratory49 but not another427 reported that E3-6.7K is required together with RID to down-regulate TRAIL receptor 1. A cytosolic tyrosine sorting motif (YXXφ) in RIDβ110,428 and a cytosolic dileucine motif 286 and tyrosine-containing motifs118 in RIDα are required for these proteins to function.
RID also has been shown to inhibit TNF-induced translocation of cytosolic phospholipase A2 to membranes (from where arachidonic acid is generated)390 and to inhibit
lipopolysaccharide- and interleukin-1β–mediated signaling responses.150 Thus, both RID and E3-14.7K might function in vivo to inhibit inflammatory responses associated with infection.
lipopolysaccharide- and interleukin-1β–mediated signaling responses.150 Thus, both RID and E3-14.7K might function in vivo to inhibit inflammatory responses associated with infection.
In contrast to most laboratories who have studied RID, one laboratory has reported that RIDα can function in the absence of RIDβ to down-regulate EGFR.118,293,645 This laboratory also reported that RIDα alone is able to regulate endosome maturation by mimicking guanosine triphosphate (GTP)-Rab7645 and to activate an autonomous cholesterol regulatory mechanism.117 Possible explanations for how RIDα, RIDβ, and the RIDα/RIDβ complex (i.e., RID) could function independently have been discussed.645
As mentioned, the E3-6.7K protein is required together with RID to down-regulate TRAIL receptor 2 from the cell surface in Ad-infected cultured cells.49,427 In transiently or stably transfected Jurkat cells, E3-6.7K alone inhibited apoptosis induced through Fas, TNF receptor, and TRAIL receptors and prevented TNF-induced release of arachidonic acid.480 Further, E3-6.7K was shown to interact with calcium modulator and cyclophilin ligand (CAML), a calcium-modulating protein,241 to prevent calcium efflux from the ER, maintain calcium homeostasis, and inhibit apoptosis induced by thapsigargin (which inhibits calcium uptake by the ER).241,480
The functions for the E3 proteins as described previously have been determined by studies in cell culture, and the question arises as to whether the E3 proteins have these same functions or other unknown functions in vivo (animal models and humans). Some and perhaps all of the E3 proteins are not essential for Ad5-based oncolytic vectors to replicate in Syrian hamsters705 or in cancer patients, but there is good evidence that E3 proteins have functions in vivo. In one study, expression of all the E3 proteins from a replication-defective Ad vector prolonged transplants of human cells in immunocompetent mice.724 In another study, the entire Ad2 E3 region stably expressed in an immortalized Gunn rat hepatocyte cell line protected against allograft rejection following transplantation into rats.458 This protection correlated with reduced Fas expression and inhibition of Fas-mediated apoptosis in the transplanted cells (which could be mediated by RID). Expression of E3-gp19K plus RID by lentivirus vectors in an insulin-producing β-cell line corrected diabetes in allogeneic mice, whereas the cell line without E3-gp19K plus RID did not protect.383 In transgenic mice, the entire Ad2 E3 region that was placed under the control of the rat insulin promoter (RIP) for targeted expression in murine pancreatic islet cells prevented autoimmune diabetes in a virus-induced (lymphocytic choriomeningitis virus) murine model of type I diabetes or in nonobese diabetic (NOD) mice.179,755 In further studies in which all E3 genes except E3-gp19K or except the E3B region genes (E3-RID plus E3-14.7K) were expressed from the RIP in transgenic mice, one study found that E3-gp19K but not the E3B region genes prevented autoimmune diabetes,310 but another study obtained the opposite result.566 In studies addressing E3-14.7K, the gene for E3-14.7K, which was cloned into vaccinia virus together with the tnf gene, increased the virulence of the vaccinia virus carrying the tnf gene alone by reversing the antiviral effects of TNF.731 In other studies, a transgenic mouse was constructed in which E3-14.7K is expressed selectively in the distal respiratory epithelium (alveolar and bronchial) from the surfactant protein B promoter.264 E3-14.7K protein suppressed pulmonary epithelial cytotoxicity and lung inflammation in response to respiratory infection with a replication-defective Ad vector or to intratracheal administration of lipopolysaccharide.264 In an influenza virus study, E3-14.7K reduced expression in the lung of CCL2, a chemokine that functions in recruitment of inflammatory monocytes and lymphocytes.678 E3 genes were required to reduce inflammation in an Ad5 pneumonia model in cotton rats227 and mice.676 The E3-6.7K plus E3-gp19K proteins were required to prolong persistence of an oncolytic Ad in Syrian hamsters.65 These various studies suggest that E3 proteins function in vivo in a manner consistent with their functions seen in vitro.
Pathogenesis and Pathology
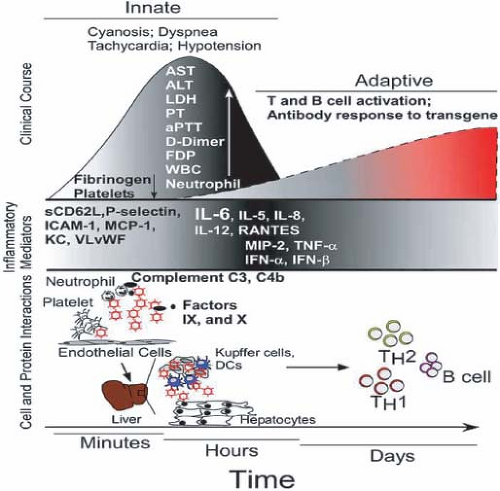
Ad infection causes inhibition of cellular DNA, mRNA, and protein synthesis. The infected cell degenerates in specific ways that help the pathologist diagnose Ad infection on biopsy or autopsy tissue.500 The respiratory epithelial cells that are affected during Ad pneumonitis have enlarged nuclei containing amphophilic or basophilic inclusion bodies surrounded by thin rims of cytoplasm. Some of these cells are referred to as smudge cells and contrast with those infected cells with nuclear inclusions but intact nuclear membranes. As in tissue culture, the epithelial cells are the primary target for Ad cytopathology in vivo.70
One of the Ad structural proteins, the penton base, made in much larger excess than needed for the assembly of Ad, binds to the av β3 and av β5 integrins via an arginine-glycine-aspartate (RGD) motif in penton base28 and causes cells to detach rapidly from monolayer cell culture dishes.780 This effect can be demonstrated even in cells that are not directly infected with Ads; it has been postulated that this protein might be involved in pathogenesis. It had been thought that the penton cell-rounding effect led to cell death, but it now appears that the cells are viable upon removal of the viral protein.508 Although the importance of penton in human disease has not been determined, it has been found in the blood of several fatal cases of Ad pneumonia.400
Ad-induced changes at the level of single cells result in considerable organ toxicity during serious tissue invasion by Ads. Necrotizing bronchitis, bronchiolitis, and interstitial pneumonia, as well as fibrin and hyaline membranes within the alveoli, characterize Ad pulmonary syndromes.168 Comparable lesions occur within the conjunctiva, in which exudative and mononuclear infiltrates are found. However, the lesions occur beneath the epithelium, so that ulceration or neovascularization of the cornea usually does not occur.335,478
Proliferative responses similar to transformation have not been recognized in acute Ad infections in humans. However, lymphatic tissue is often hypertrophied, and active germinal centers are found. For example, the appendices of children undergoing surgery to correct bowel obstruction found in intussusception may be associated with enlarged mesenteric lymph nodes and Ad isolation from stool specimens.807 These lymphocyte changes are often in close proximity to areas of desquamated epithelial cells in which typical viral inclusions may be found. The inclusions contain viral particles in crystalline arrays that are visualized by electron microscopy (EM). Some of the lymphocytes are probably CD8+ CTLs that recognize proteolytic peptide products of viral proteins.
Entry into the Host
Ads enter susceptible hosts by the mouth, nasopharynx, or ocular conjunctiva. Recent experiments have defined the Ad receptors (Fig. 56.5) (reviewed in406,469,521,770,817). The cell protein named CAR (coxsackie-adenovirus receptor) is a receptor shared by these two groups of unrelated viruses and is the target of fiber in many if not all serotypes in species A, C, D, E, and F.55,55,597 CAR is present on tight junctions of polarized cells where it mediates cell–cell adhesion. Species B Ads can be differentiated based on their receptor usage.732 Species B, group 1 (Ad16, 21, 35, 50) viruses nearly exclusively utilize CD46 as a receptor.253,457 Initially, Ad37, a species D Ad, was also thought to use CD46,791 but recent data suggest that GD1a glycan is the more likely major receptor for this virus.525 CD46 (also known as membrane co-factor protein) is expressed in virtually all cells where it acts as a co-factor for inactivation of the complement components C3b and C46. Species B, group 2 (Ad3, Ad7, 14) viruses share desmoglein 2 as the high-affinity receptor.769,770,771 Desmoglein 2 is a calcium-binding transmembrane glycoprotein belonging to the cadherin protein family. When the Ad fiber binds to desmoglein 2, there is opening of intercellular junctions that results in increased access to receptors trapped deep within the junction (i.e., CD46 and Her2/neu).770 Further, such dissociation of the intercellular junctions may facilitate the lateral viral spread in epithelial cells and, potentially, the penetration of Ad into subepithelial cell layers and the bloodstream. Lastly, species B, group 3 (Ad11) viruses preferentially interact with CD46 but also utilize desmoglein 2 if CD46 is blocked.770
Heparin sulfate glycosaminoglycans, which are long heterogeneous, heavily sulfated carbohydrates that are abundant within the extracellular matrix, can mediate the CAR-independent binding of Ad2 and Ad5 to cells, but it is not known if these are bona fide receptors.817 The class I MHC molecule has been proposed as a second receptor for Ad5304 but has not been confirmed by other groups.142,463 Three species D Ads, Ad8, Ad19, and Ad37, all major causes of EKC, appear to bind to α2,3-linked sialic acid present in the GD1a ganglioside on the corneal cell surface.525 Several integrins participate in Ad uptake into cells by interacting with the RGD motif on the Ad penton base protein.817
Most of the studies described previously were conducted in vitro. Recent studies of systemic administration of Ad vectors in mice raise the possibility that infection of tissues in vivo may be different (see section on Adenoviruses as Vectors for Vaccination and Gene Therapy). These studies propose a CAR-independent model in which Ad infection of murine hepatic cells occurs through binding of Ad to blood factors, especially factor X, directing the complexes to hepatocellular receptors including heparin sulfate proteoglycans (Fig. 56.5). Whether this pathway operates in humans is a key281 question. Recent studies clearly demonstrate that all of the tested species of Ads, except for the species D viruses, efficiently bind human factor X.757 The high-affinity interaction of Ads with factor X may facilitate “bridging” the hexon protein in the Ad capsid to heparin sulfate proteoglycans expressed on the surface of hepatocytes.352,757 The Ad–factor X complex binds to the cell surface through the serine protease domain of factor X and not through a direct interaction of the virus with the cell surface.757 Once bound to the cell surface, efficient and rapid intracellular transport of the Ad, though, remains dependent upon engagement of αv integrins via the penton base protein.71
The lower serotypes, Ad1, 2, 5, and 6, are ubiquitous, particularly in young children, and may be shed for months, especially in the stool, which is probably responsible for the endemic spread of these agents by the fecal–oral route to new pools of susceptible infants and children.205 An epidemic form of Ad keratoconjunctivitis (EKC) has been spread in swimming pools207 from contaminated water and in medical settings336 from Ad-infected ophthalmologic instruments. A third pattern of spread was unique in the military setting, occurring during the early period after induction into service. Ad4 and Ad7 caused acute respiratory distress (ARD), including pneumonia, and were the result of air-borne inoculation of the respiratory tract168 as well as acquisition after contact with contaminated surfaces in living quarters.615
Site of Primary Replication
It is clear from the original findings of Ads in tonsils and adenoids that these tissues of the oropharynx are a major initial site of replication for the entering Ads. For serotypes causing
respiratory disease, the initial replication most likely occurs in the nonciliated respiratory epithelium, although some limited replication and persistence can also occur within lymphocytes (reviewed in445). These conclusions are based not on careful observations of cells in situ but on the cells in which Ad replicates in tissue culture. Ciliated respiratory epithelium of the lower airway is difficult to infect with Ads through the apical surfaces, which do not contain the CAR receptor; however, disruption of the integrity of cell–cell contact allows basolateral infection of such polarized epithelial cells using CAR.565,768
respiratory disease, the initial replication most likely occurs in the nonciliated respiratory epithelium, although some limited replication and persistence can also occur within lymphocytes (reviewed in445). These conclusions are based not on careful observations of cells in situ but on the cells in which Ad replicates in tissue culture. Ciliated respiratory epithelium of the lower airway is difficult to infect with Ads through the apical surfaces, which do not contain the CAR receptor; however, disruption of the integrity of cell–cell contact allows basolateral infection of such polarized epithelial cells using CAR.565,768
Spread of Virus and Tissue Tropism in the Host
Most of the manifestations of Ad infection are locally in the eyes and pharynx, but contiguous extension into the lungs results in some cases. One possible molecular mechanism facilitating the spread of the virus over respiratory epithelia is that the fiber protein, which is synthesized in great excess in the infected cell and is released when the cell is lysed, binds CAR on the basolateral surface. This binding disrupts the CAR homodimers in tight junctions, thus increasing paracellular permeability. This in turn allows the virus to escape onto the apical surface of the respiratory epithelium, thereby making it possible for the virus to infect other areas of the respiratory tract.767 Likewise, for the species B viruses that bind to desmoglein 2, binding to desmoglein 2 resulting in dissociation of the intercellular junctions may facilitate spread of the virus locally and into deeper tissue layers, allowing access to the bloodstream.770 Even in the nonreplicating mouse model of human Ad infection, the virus can cause inflammation in the lungs, which is strong evidence for direct extension into these target organs.228,676 Ads have been cultured from the blood during fatal Ad respiratory disease, suggesting viremic spread in some situations (see the section later on immunocompromised patients). As CAR is present on endothelial cells, viremia might be promoted by the fiber protein through the mechanism described earlier. The successful use of oral, live, microencapsulated Ad vaccines by the military to prevent ARD suggests that if the respiratory tract can be physically bypassed by Ad4 and Ad7, intestinal replication of the virus causes an immunizing rather than a virulent infection.129 Although most Ads replicate in the intestine without causing gastroenteritis, Ad40 and 41 are responsible for intestinal disease. Ad disease in the urinary bladder, primarily by species B Ads in immunocompetent hosts, suggests that the virus probably is viremic at some stage in order to reach this organ. There is no evidence for ascending infection for these species B serotypes, which are less commonly found in the intestine and are more common in young males than in females. The route of infection of the liver, especially in immunosuppressed liver transplant recipients, is unknown, but some patients might be infected by latent Ads that are present in cells such as lymphocytes in the transplant.
Immune Response
Various aspects of the immune response to Ads have been described in other sections, as have mechanisms that the virus uses to evade the immune response (see Fig. 56.3 and section on Functions of Adenovirus E3 Proteins). The innate response to Ad also has been reviewed439,504 (see section on Adenoviruses as Vectors for Vaccination and Gene Therapy). In the airways, the virus must penetrate the surface fluid, and sialic acid present in the mucus may bind and inhibit species D Ads that use sialic acid as a receptor.342,525 The virus must also survive chemical defenses of the host. These include a large variety of antimicrobial peptides that are able to neutralize microbes directly. Among these peptides are the defensins, a family of small cationic amphipathic peptides divided into two classes, α-defensins and β-defensins (reviewed in273). The α-defensins HNP1 and HD5 were shown to neutralize Ad serotypes in species A, B1, B2, C, and E, but not in species D and F.270,671,672 The defensins bind Ad particles outside the cell, block uncoating of the virion, and restrict the release of virions from endocytic vesicles.671 Thousands of molecules of α-defensins bind to a sensitive serotype; neutralization of the virus depends on binding to critical determinants in a region spanning the fiber and penton base proteins.672 Binding to these determinants is proposed to prevent the release of fiber from the virion, the first step in the virion uncoating process within the endosome.672 HNP1 is expressed primarily in neutrophils, monocytes, lymphocytes, and natural killer cells, while HD5 is expressed mainly by Paneth cells in the intestine.273,671 It is not known if these or other defensins play a role in Ad infections in humans.
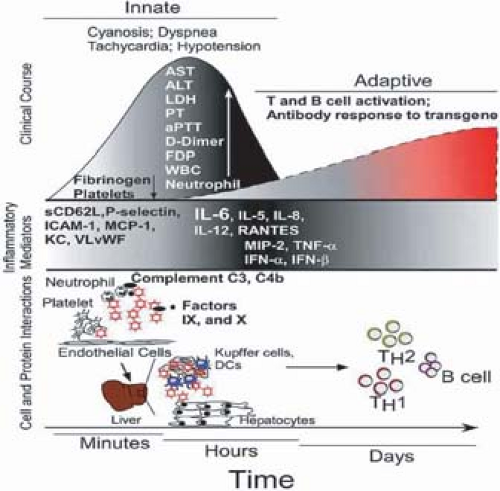
With respect to cellular defenses, alveolar macrophages and Kupffer cells play an important role in elimination of Ad vectors from the lung and liver, respectively, in murine models439,504 (Fig. 56.6) (see section on Adenoviruses as Vectors for Vaccination and Gene Therapy). These cells take up the vectors rapidly and secrete inflammatory cytokines such as TNF, interleukin-6 (IL-6), and IL-8. A robust inflammatory response characterized by early cytokine (IL-1β, IL-6, interferon-γ [IFN-γ], IL-12, and TNF) and chemokine release as well as neutrophilic and monocytic infiltration have been described in the murine pneumonia model after nonpermissive infections with species C Ads.228,349 Increased levels of IL-6, IL-8, and TNF have also been associated with Ad infection in children.476 A recent study suggested that there may be specific cytokine signatures in pediatric stem cell transplant recipients with localized and invasive Ad infection. Patients with invasive infections had increased levels of the pro-inflammatory cytokines IL-1β, IL-6, IL-8, IL-12, IFN-γ, and TNF-α, as well as IL-17, macrophage inflammatory protein-1α (MIP1α), oncostatin M (OSM), and IFN-γ–inducible protein 10 (IP10).272 Invasive Ad infection was also associated with the simultaneous release of the cytokines IL-1β, IL-17, IL-18, OSM, MIP-1α, and IP10.272
Ad infection of cultured cancer cells, respiratory epithelial cells, hepatocytes, and antigen-presenting cells activates the NF-kB and MAP kinase pathways.547 Induction of IL-6 and IL-8 does not require Ad gene expression and likely occurs via interaction of the virion with integrins on the cell surface. A biphasic mononuclear cell response in the cotton rat suggested an early appearance of monocytes and NK cells followed by specific T and B lymphocytes. The cell infiltrate was changed from a mononuclear to a polymorphonuclear leukocyte response by deletion of the Ad E3 anti-TNF genes (14.7k, ridα, ridβ). Deletion of the anti-MHC Ad E3-gp19K protein increased the intensity of the inflammatory response in the cotton rat but not in the mouse.227,676 These observations suggest that immunopathology plays a role in the host response to
Ad infection, and this process is ameliorated by the E3 inhibitors of the host inflammatory response.227,574 Another Ad protein, the L4-100K protein, which is expressed at late stages of infection, inhibits granzyme B–mediated death by CTL.16 Thus, it is not clear at this point whether the cell-damaging effects of Ad infection or the host immune responses to the pathogen are responsible for the tissue pathology and clinical manifestations.
Ad infection, and this process is ameliorated by the E3 inhibitors of the host inflammatory response.227,574 Another Ad protein, the L4-100K protein, which is expressed at late stages of infection, inhibits granzyme B–mediated death by CTL.16 Thus, it is not clear at this point whether the cell-damaging effects of Ad infection or the host immune responses to the pathogen are responsible for the tissue pathology and clinical manifestations.
Induction of type I interferons (α and β) is part of the innate response to Ad infection. Ads have at least two mechanisms to counteract these interferons (see Chapter 55). First, the E1A proteins block the assembly of interferon-induced transcription factors, and second, VA-RNAI, a 159-nucleotide RNA, binds to the protein kinase named PKR, prevents its activation by double-stranded RNA, and thereby prevents PKR from phosphorylating eukaryotic initiation factor eIF2 and shutting down protein synthesis.
Neutralizing and nonneutralizing antibodies are discussed in the section, Description of Key Antigens. After infection, most patients develop both group- and type-specific antibodies to the infecting Ad strain. Group-specific antibodies are not neutralizing of viral infectivity but are useful in measuring patient response when infection with an Ad is suspected but no isolate was obtained. In this case, acute and convalescent sera taken several weeks apart may demonstrate rising antibodies to a group-specific Ad antibody such as that measured by CF, IF, or ELISA, using a viral-infected tissue culture extract or purified hexon from a single Ad type. Type-specific antibodies are measured by either the neutralization test or inhibition of HA, as described in the section Viral Serology. It is clear that patients may continue to shed Ads intermittently, especially in their stools, for many months after a successful humoral immune response has occurred.205 Neutralizing antibodies may be protective against disease manifestation in the previously infected host or against reinfection with the same serotype, but they do not eliminate the carrier state.
Recent studies have begun to define the T-cell response to Ad infection in humans and Ad (see the section on Description of Key Antigens) (reviewed in382,409,762). Although most of the early work in humans described Ad-specific CD4+ T cells, recent studies have detected and characterized Ad-specific CD8+ T cells. These CD4+ and CD8+ T cells are frequently cross-reactive among Ad serotypes, and many of the epitopes have been mapped to the conserved regions of hexon.
Although it is not known if these T-cell epitopes are protective against Ad in humans, most likely T cells in general are protective because the absolute lymphocyte level and the CD4+ T cell level correlate inversely with Ad infection and disseminated adenoviremia in immunosuppressed transplant patients (see section on Diseases Associated with Immunocompromised Patients). For example, in adult stem cell transplant recipients, clearance of human Ad viremia coincides with emergence of a coordinated CD8+ and CD4+ T-cell response against Ad hexon epitopes.199,277,813
Although donor lymphocyte infusions can be used in hematopoietic stem cell transplant recipients with Ad infections to improve infectious diseases outcomes, potentially life-threatening graft-versus-host disease (GVHD) may develop.125 Adoptive transfer of CTL against Ad can rapidly reconstitute anti-Ad immunity after stem cell transplantation without causing GVHD.199,277,410,813 Methods are being developed to activate and expand T cells in vitro, and these interventions appear to be a promising potential therapy for immunocompromised patients with serious Ad infections (reviewed in5,367,409,412,474,813).
Virulence
It is not known why certain serotypes characteristically cause disease in some organs and not in others. Tissue culture experiments and the existing animal models do not explain the mechanisms of such tissue tropism or organ-specific pathogenicity. There is nothing obvious about the bioinformatics analysis of multiple Ad genomes that explains differences in pathogenicity, although most differences among species are in the E3 region.141 Because most Ads can attach to the CAR receptor, it is unlikely that this receptor can explain differences in the in vivo tropism of the various serotypes. Even the oral vaccine strains used to prevent respiratory disease in military personnel seem to depend more on physical formulation (i.e., enteric coating of a live virus so that it bypasses the oropharynx and first exposes the intestinal epithelium) than on mutation of virulence genes.446
Epidemiology
Human Ad serotypes are generally not pathogenic to animals, and animal Ads are pathogenic only within the species of origin.702 However, simian species occasionally have been shown to have antibodies to human Ad12,353 and antibodies to simian Ads have been detected in human sera.187 Recently, a novel Ad named titi monkey Ad, which caused a deadly outbreak of pneumonia and hepatitis in a closed colony of titi monkeys in California, caused a respiratory infection in a researcher in close contact with the monkeys, and this infection spread to a family member.105 A screen of 81 random adult blood samples revealed neutralizing antibodies to this virus in 2 individuals. The native species for this virus is unknown. Of added interest, Ads isolated from great apes are phylogenetically related to human Ads in species B, C, and E, consistent with the possibility of interspecies spread at some point in time; however, frequent interspecies infections are unlikely.614
In humans, transmission of Ad infection and disease varies from sporadic to epidemic. The pattern often correlates with the viral serotype and the age (children or adults) of the susceptible population. A virus watch study was undertaken in New York and Seattle,205,206 and much of the Ad epidemiology in civilian populations was learned from these studies, including that there was a high incidence of recurrent shedding, especially of the lower numbered serotypes, in fecal specimens. A large number of asymptomatic Ad infections were documented. Fecal–oral transmission accounts for most infections in young children.43 Initial spread may occur by the respiratory route, but the prolonged carriage in the intestine makes the feces a more common source during both the acute illness and intermittent recurrences of shedding.205 The epidemiologic importance of the long latency in tonsil tissue is not known.
Ads are estimated to cause 8% of clinically relevant viral disease globally. They probably account for 3% of the infections in civilian populations and for about 5% to 10% if only febrile illnesses are calculated.205,206 The corresponding figures for young children are about 5% and 10%, respectively. In a recent PCR-based multicenter study, 4.4% of pediatric patients with diarrhea in Asia were shown to have Ad-positive stool samples, with Ad40 and 41 being the most prevalent serotypes.424 According to a survey carried out in Manchester, United Kingdom, 61.3% of patients with Ad infection were younger than 5 years of age, 24.2% were adults, and 5.6% were children between 5 and 18 years old.126 The most prevalent serotypes were Ad2 (18.6%), Ad3 (14.9%), Ad1 (12.1%), and Ad41 (10.9%). While Ad2, 1, and 41 were isolated mostly from infants, Ad3 and, less frequently, Ad4 (8.3%) was recovered from adults.
Serologic surveys have furnished estimates of the prevalence of Ad infections. Early surveys indicated that antibodies to Ad1, 2, and 5 are most common and are present in 40% to 60% of children.27,73,317 The incidence of antibodies to Ad3, 4, and 7 is low at the same ages. These antibody results probably explain why adults are uncommonly infected with Ad1, 2, and 5 but are more susceptible to infections with Ad3, 4, and 7. During the surveillance for the virus watch studies, only about 75% of the Ad isolates were accompanied by an antibody response, as measured by the CF test.205
More recent surveys have provided additional epidemiologic information. Ad5 neutralizing antibodies have been found in ∼80% to 90% of the population in Sub-Saharan Africa.1,36,106,167,301,332,459,533,693,710 In one study of sera from Africa, the seroprevalence of Ad5 was very high in neonates (93%, with 48% having neutralizing titers of greater than 1,000), and it correlated with maternal titers.710 Interestingly, the seroprevalence was 13% in subjects aged 6 months to 1 year, was 28% at age 1 to 2 years, and then reached high adult levels at age 7. These data suggest that neonates acquired maternal antibody, which then declines following birth, but the children acquire new infections as they age.710 Seroprevalence for Ad5 is 40% to 70% in Japan,301,754 ∼85% in China (54% with neutralizing titers greater than 1,000),694 70% to 80% in Brazil (with 14% greater than 1,000 neutralizing titers),187,459 nearly 100% in northern India with 25% of individuals having high neutralizing titers (greater than 1,000) and 31% having very high titers (greater than 10,000),567 and 82% to 94% in Thailand.36,459 In the United States, Ad5 seroprevalence ranges from 30% to 70% in various studies.25,36,108,301,459,533,627,754 In Europe, the seroprevalence of Ad5 is 50% to 60%.301,385,459,754 Ad6 (species C) is being evaluated as a vaccine vector263,776; overall in the world, about half of subjects have neutralizing antibodies to Ad6, but mostly of low titer.138,459,624 For Ad2, which is closely related to Ad5 and Ad6 in structure and clinical properties, the seroprevalence was found to be 83% in one study of subjects from Philadelphia.25
Seroprevalence studies have also been conducted for more rare serotypes, motivated in part because of considerations to use these serotypes as vectors. For Ad35 (species B), the seroprevalence was estimated to be between 025 and 10%36,533,754 in the United States, less than 10% in Europe,385,754 ∼15% in Japan754 and Thailand,36 and ∼3% to 20% in Sub-Saharan Africa.36,385,533 With Ad11 (species B), seroprevalence is 10%710 to 30%1,301 in Sub-Saharan Africa and Japan. Ad26 (species D) seroprevalence is 10% (with low titers) in the United States106,459; 44% in Brazil187; 10%106 and 50% in Thailand459; and ∼20%,1 ∼50%,36,459 and 60% to 80%106 in Sub-Saharan Africa. Thus, Ad26 is not a rare serotype. Ad49 and Ad50 are rare in most areas of Sub-Saharan Africa, but Ad48 is more common in East Africa.1,36,710 With Ad36 (species D), which has been linked to increased obesity, neutralizing antibodies are rare in the United States and Thailand but were found in about half the population in Brazil and parts of Sub-Saharan Africa.459 The Ad28 seroprevalence is less than 10% in the United States.347
It should be noted that the serologic studies described previously refer primarily to neutralizing antibodies. Generally, the prevalence of total antibodies against the Ad is higher. Further, nearly all adult humans contain T lymphocytes, primarily CD4+ but also CD8+ specific for Ads.84,106,320,322 These T-lymphocyte analyses do not identify the serotype (by definition) to which they are specific, and the T lymphocytes appear to be broadly reactive because of cross-reactive epitopes (often on hexon).
The epidemic forms of Ad disease were studied in different ways from the sporadic endemic occurrences. The epidemics of ARD were well known during World War II, and this awareness preceded the isolation and the characterization of the first Ad by about one decade. This ARD, which occurred almost exclusively in recently assembled military recruits, was most common in winter. It did not occur in senior personnel in close contact with the recruits and was later identified as an Ad4 or Ad7 infection in most outbreaks (reviewed in287,575). This disease rarely occurred in similarly congregated college students, suggesting that additional factors, such as more crowded sleeping conditions or the fatigue associated with basic training, contributed. The observation that ARD-causing Ads did not spread to civilian personnel in contact with the military supports these conditions as co-factors. In recruits congregated during the summer months, ARD often did not occur until the onset of colder weather in the fall. Influenza A could be distinguished because it affected both experienced and new recruits. Ad-induced ARD often affected 80% of the recruits, with 20% to 40% hospitalized. The duration of infectivity was rather short; virus was not demonstrable after 4 days of illness.612 Controlled studies of routes of infectivity for the ARD-causing Ads have demonstrated that aerosolized virus inhaled into the lungs of volunteers produced the disease, whereas application to the mouth, the nasal mucosa, or the intestine in enteric-coated capsules failed to produce the lower respiratory disease.129 ARD outbreaks were effectively controlled by vaccination of recruits in the first few days of military service; however, recent interruption in the supplies of Ad vaccine has resulted in the reappearance of the epidemic form of ARD. This is discussed further in the section on Adenoviruses as Vectors for Vaccination and Gene Therapy.
The epidemiology of pharyngoconjunctival fever and keratoconjunctivitis, both of which may occur in epidemic proportions, is described later with the individual disease entities. Infection resulting in several of the Ad syndromes can be acquired in hospitals and can be spread as nosocomial infections.
Clinical Features
Ads can commonly infect and replicate at various sites of the respiratory tract as well as in the eye and gastrointestinal tract. Less frequently, Ads can infect the urinary bladder and liver. On occasion, these viruses may also cause disease in other organs, such as the pancreas,524 myocardium, or central nervous system, which may be involved in meningoencephalitis. Although there are at least 57 distinct human Ad serotypes, most human disease is associated with only one-third of these types.595 Many Ad infections are subclinical and result in antibody formation that probably is protective against exogenous reintroduction of the same Ad serotype. However, the virus itself may be grown, especially from the gastrointestinal tract205 and respiratory tract,189 for months after the initial infection and immune response. The more common illnesses associated with various Ads are described in Table 56.1 and in the following paragraphs. The association of Ad with the disease in question is often attributed to the detection of the virus or antibodies to the virus in the blood, or to the detection of the virus in specific tissue. Often very sensitive techniques are used such as nested PCR. It is important to bear in mind that the presence of the virus or viral DNA in clinical specimens does not necessarily imply a cause-and-effect relationship between the virus and the disease, especially when PCR or nested PCR has been used. We do not understand whether and how Ad persists at very low levels in humans, so it is possible that the detection of Ad is merely a coincidence. On the other hand, the detection of the virus should not be disregarded.
Respiratory Diseases
Endemic Adenovirus Infections of Young Children
About 7% of upper respiratory tract infection cases in children younger than 5 years of age are due to an Ad.557 The usual symptoms include nasal congestion, coryza, and cough. Other patients may have an exudative tonsillitis that may be clinically indistinguishable from disease caused by the group A streptococcus. The respiratory symptoms are often accompanied by systemic manifestations, such as generalized malaise, fever, chills, myalgia, and headache. The common serotypes are Ad1, 2, 5, and 6 (species C or HA group III) and occasionally Ad3 and Ad7 (species B or HA group I), which are endemic in most populations. Sporadic cases may be indistinguishable from other viral respiratory infections, such as influenza, parainfluenza, and respiratory syncytial virus.557 If conjunctivitis accompanies the signs and symptoms already described, the disease is designated as pharyngoconjunctival fever. The Ad serotype most commonly involved is Ad3, but Ad7 and Ad14 within the same HA group have been isolated from such patients.308
Ads also cause lower respiratory tract infections in children and are probably responsible for about 10% of the pneumonias of childhood.455,557 Most patients recover, but some epidemics have resulted in considerable mortality. Sequelae in those who recover may include bronchiectasis that can clinically manifest years after the primary infection. Ad7, in particular strain Ad7h in recent years, has been a problem in South America for pediatric lower respiratory tract infections.87 In one retrospective study in Buenos Aires, Argentina, Ad7h was associated with 29 (2.4%) of 1,233 cases (mean age 8.8 months) with a mortality rate of 34.5% between 1984 and 1988.497 In another study of 22 cases in 1991–1992 in Buenos Aires, Ad7 was found in 82% of patients (12 of 14 were Ad7h) and species C in 18% of patients.87 Four patients died, three of whom had Ad7h. In a similar study in Uruguay in 1994–1998, 32% of pediatric patients (mean age 8.8 months) had species B (all but one Ad7h) and 61% had species C.208 In a Taiwan study in 1999–2000, nine children (mean age 22 months) had Ad3, one had Ad2, and one had Ad11.115 In a U.S. study, there was a 6-month outbreak of Ad30 (species D) in a neonatal intensive care unit that involved 21 of 333 patients (6.3%) and that had pneumonia in 8 patients.190 Six infants died, and death was associated with pneumonia.
Acute Respiratory Disease in Adults
In adults, the serotype subspecies B1 Ads (Ad3, Ad7, Ad16, Ad21, and Ad50) and species E (Ad4) are commonly associated with acute respiratory disease, whereas subspecies B2 viruses
(Ad11, Ad14, Ad34, Ad35) are more frequently associated with urinary tract and opportunistic infections in immunocompromised patients. Without significant circulation in North America, Ad14 emerged as a significant cause of acute and sometimes severe acute respiratory disease in 2006. Initially, the infection was recognized in three military bases under continuous systematic surveillance,471 but widespread outbreaks were demonstrated shortly thereafter in Washington, Oregon, Alaska, Wisconsin, and Pennsylvania, and to a lesser extent in other states.92,93,188,313,350,421,441,701 The DNA sequence of the prototype Ad14 (de Wit strain, isolated in 1955) was published recently.643 Sequencing of the outbreak strain e1a, hexon, and fiber genes suggests that Ad14p1 arose from recombination among similar Ad11 and Ad14 ancestral strains. A deletion of two amino acids in the knob region of the fiber protein is the only identified unique characteristic of Ad14p1.350 Low antibody titers against Ad14 in recruits sampled at admission to training camp and initial detection of Ad14p1 in cities on the U.S. West Coast that represent major ports of entry into the country from Asia suggest that travel and commerce played a major role in the introduction of this rare Ad subspecies B2 virus into the United States.350,701 Current studies are ongoing to better understand why the virus resulted in significant morbidity and mortality in a small subset of infected individuals; in one of the earliest outbreaks, in Oregon, there were 29 hospitalizations with 7 associated deaths.350,421 During the investigation of this initial civilian outbreak, 67 cases of Ad infection were detected during the study period and 40 (60%) involved Ad14.421 Of those with medical records available for review, most presented with fever and cough; 29 (76%) required hospitalization, 23 (61%) required supplemental oxygen, 18 (47%) required critical care, 9 (24%) required vasopressors, and 7 (18%) died. Older age, chronic underlying condition, low absolute lymphocyte counts, and elevated creatinine levels were associated with severe illness. In most instances, no epidemiologic link between cases could be established.188,421
(Ad11, Ad14, Ad34, Ad35) are more frequently associated with urinary tract and opportunistic infections in immunocompromised patients. Without significant circulation in North America, Ad14 emerged as a significant cause of acute and sometimes severe acute respiratory disease in 2006. Initially, the infection was recognized in three military bases under continuous systematic surveillance,471 but widespread outbreaks were demonstrated shortly thereafter in Washington, Oregon, Alaska, Wisconsin, and Pennsylvania, and to a lesser extent in other states.92,93,188,313,350,421,441,701 The DNA sequence of the prototype Ad14 (de Wit strain, isolated in 1955) was published recently.643 Sequencing of the outbreak strain e1a, hexon, and fiber genes suggests that Ad14p1 arose from recombination among similar Ad11 and Ad14 ancestral strains. A deletion of two amino acids in the knob region of the fiber protein is the only identified unique characteristic of Ad14p1.350 Low antibody titers against Ad14 in recruits sampled at admission to training camp and initial detection of Ad14p1 in cities on the U.S. West Coast that represent major ports of entry into the country from Asia suggest that travel and commerce played a major role in the introduction of this rare Ad subspecies B2 virus into the United States.350,701 Current studies are ongoing to better understand why the virus resulted in significant morbidity and mortality in a small subset of infected individuals; in one of the earliest outbreaks, in Oregon, there were 29 hospitalizations with 7 associated deaths.350,421 During the investigation of this initial civilian outbreak, 67 cases of Ad infection were detected during the study period and 40 (60%) involved Ad14.421 Of those with medical records available for review, most presented with fever and cough; 29 (76%) required hospitalization, 23 (61%) required supplemental oxygen, 18 (47%) required critical care, 9 (24%) required vasopressors, and 7 (18%) died. Older age, chronic underlying condition, low absolute lymphocyte counts, and elevated creatinine levels were associated with severe illness. In most instances, no epidemiologic link between cases could be established.188,421
Acute Respiratory Disease of Military Recruits
In many respects, ARD is similar to the description furnished earlier of the respiratory infection of children. The syndrome is predominantly caused by Ad4 (92.8% in one study conducted in 2004–2006),244 and less commonly Ad7. After 2005, coincident with similar findings in the civilian populations, there was the simultaneous emergence of increased diversity of infections due to subspecies B1 serotype 3, 7, and 21 and subspecies B2 serotype 14.471 ARD is a syndrome that frequently occurs under the special conditions of fatigue and crowding created soon after the induction of young military recruits (reviewed in308,575). Some cases have had a fatal outcome from the pneumonitis that may accompany and complicate the other, milder respiratory symptoms. A significant increase in severe and fatal cases was seen recently with the emergence of Ad14 infections in two U.S. military facilities.471,518 On the other hand, recent retrospective studies of Air Force recruits with pneumonia at Lackland Air Force Base did not find evidence that Ad14 was associated with excess overall morbidity as compared to pneumonia due to other Ad serotypes.752 Further aspects of this disease are discussed in the sections entitled Epidemiology and Vaccines.
Pertussis-Like Syndrome
The association of Ad infection with a pertussis-like syndrome has led to speculation that Ads can cause clinical whooping cough.689 Ad5 was isolated from multiple organs of a patient with severe whooping cough with lymphocytosis that ended fatally.124 Later data from a controlled study of 134 children with a pertussis-like illness and 101 healthy controls reported the common association of Ads with whooping cough symptoms.506 Ads have been isolated from other studies of respiratory tract infections including pertussis-like syndrome.195,586 However, there was no evidence that the Ads alone were responsible for the syndrome. The large number of Ad isolates may be due to conditions favorable for reactivation of latent viruses from tonsillar tissue during concurrent B. pertussis infection.516
Infections of the Eye
An acute follicular conjunctivitis may occur as part of a respiratory-pharyngeal syndrome or as a separate entity.53,496 Both bulbar and palpebral conjunctival involvement may occur and affect both eyes. The disease is often accompanied by significant preauricular lymphadenopathy.44 Complete recovery without sequelae is the most common result of this rather mild illness. The incubation period is usually 6 to 9 days, but it was as short as 2 days in experimental infections in volunteers.46,348 Epidemiologically, these infections can occur sporadically or cause disease in large groups of contacts. Family members may be affected. When the source is a swimming pool or small lake, large numbers of children and young adults may develop symptoms. Swimming pool conjunctivitis is probably most commonly due to Ads.207 Although the virus is isolated from the conjunctiva of affected individuals, it has not been isolated from water samples from putatively infected sources. The common-source water-borne outbreaks usually occur in summer and are caused by Ad3 and Ad7; however, other types, such as Ad1, 2, 4, 6, 9 to 11, 15 to 17, 20, and 22 (species B, C, D, and E and HA groups I, II, and III), have been associated with this syndrome.53,496
In contrast to the milder form of ocular disease described previously and limited to the conjunctiva, EKC is a highly contagious and more serious disease. The clinical entity was first described before the isolation of Ads and occurred among German workers in the late 19th century.299,334 It was subsequently observed in shipyard workers in Hawaii and in the continental United States. “Shipyard eye” was probably transmitted in the medical facilities that cared for chemical and physical trauma to the eyes of the workers. After an 8- to 10-day incubation period, a follicular conjunctivitis with edema of the eyelids, pain, lacrimation, and photophobia began. Corneal subepithelial infiltrates often followed the initial conjunctival involvements.143 The disease is often unilateral with preauricular lymph node hypertrophy. Occasionally, other lymph nodes were involved, and constitutional symptoms occurred, especially in children. However, most often the disease was limited to the involved eye and its draining lymph nodes. Corneal opacities in some cases lasted for several years and, uncommonly, would remain for longer periods. In some patients, the involvement slowly progressed to a hemorrhagic conjunctivitis that should be distinguished from the rapidly evolving acute hemorrhagic conjunctivitis associated with enterovirus 70.143 In addition to viral inoculation during an ophthalmologic procedure such as tonometry, EKC has occurred as a late summer and fall epidemic in certain parts of Japan, Taiwan, and Vietnam. Ad8 was the original cause of EKC, but outbreaks of Ad3, Ad19, Ad34, Ad37, Ad53, and Ad54 (a novel hexon-chimeric intermediate Ad22,37/H8)
have also been described.18,23,47,97,103,327,360,365,561,699,704 Recent outbreaks of EKC between 1995 and 1997 were caused by a unique isolate, designated Ad8I, which has Ad9 sequences in the hypervariable region of the hexon gene that may have allowed the virus to escape pre-existing neutralizing antibodies to Ad8 in the population.22 Treatment of severe infections caused by Ad8 with N-chlorotaurine, an antimicrobial agent, shortened the duration of illness and was well tolerated.704
have also been described.18,23,47,97,103,327,360,365,561,699,704 Recent outbreaks of EKC between 1995 and 1997 were caused by a unique isolate, designated Ad8I, which has Ad9 sequences in the hypervariable region of the hexon gene that may have allowed the virus to escape pre-existing neutralizing antibodies to Ad8 in the population.22 Treatment of severe infections caused by Ad8 with N-chlorotaurine, an antimicrobial agent, shortened the duration of illness and was well tolerated.704
Acute Hemorrhagic Cystitis
Acute hemorrhagic cystitis, an illness occurring almost exclusively in boys and associated with Ad11, is characterized by gross hematuria.530 Its significance lies in the potential confusion with other, more serious diseases of the kidney (such as glomerulonephritis). This self-limited disease is usually not accompanied by fever or hypertension, and tests of renal excretory and concentrating functions have been essentially normal. Ad21, like Ad11, is a species B HA type I and can also cause hemorrhagic cystitis.493 In Japan, when acute hemorrhagic cystitis occurs in a boy between the ages of 6 and 15 years, an Ad isolation from urine or a rise in neutralizing antibody occurs in about 70% of patients.530 In comparable studies of hemorrhagic cystitis in the United States, only 20% of cases can be linked to an acute Ad infection, and for 60% of the total, the etiology remains unexplained.493
The other population that develops hemorrhagic cystitis is immunosuppressed transplant recipients. According to one study, Ad infections account for 3.9% of hemorrhagic cystitis among pediatric hematopoietic stem cell transplant (HSCT) recipients.235 Another study of mostly adult HSCT recipients found that Ad was associated with 9.8% of cases of hemorrhagic cystitis.486 Ad hemorrhagic cystitis in HSCT patients is more frequently associated with T-cell purging and was less common in patients with acute GVHD than other causes of hemorrhagic cystitis.486 In the kidney transplant population, Ad can also cause hemorrhagic cystitis with or without concomitant nephritis; such patients typically present with fever and often feel poorly.12,261,282,380,544 In some patients, it appears that the infection was introduced with the transplanted kidney.389,746 In addition to Ad11, two other species B serotypes, Ad34 and Ad35, were isolated first from renal transplant recipients. Ad34 was isolated from urine, whereas type 35 was isolated from kidney and lung tissue at autopsy.283,500 Although neither was accompanied by the symptoms of hemorrhagic cystitis, the Ad35 clearly contributed to the patient’s demise from pneumonia. Ad34, 2, and 31 were isolated from allogeneic bone marrow, hematopoietic stem cell, and liver transplant recipients with hemorrhagic cystitis, respectively.485,632
Meningoencephalitis
It is rare to isolate any of the Ads from either the cerebrospinal fluid (CSF) or the brain. However, several reports have directly demonstrated Ads in CSF (Ad3, 5, 6, 7, 7A, 12, and 26).113,166,364 One patient with malignant lymphoma, immunosuppressed by chemotherapy, had an Ad32 isolated from the brain at autopsy.606 A patient with large B-cell lymphoma developed meningoencephalitis due to Ad7.200 Ad5 was cultivated from CSF in two immunocompetent patients, one with meningoencephalitis and the other with meningitis.674 A bone marrow transplant developed fatal subacute Ad meningoencephalitis.140 A 12-year-old immunocompetent girl developed tubulointerstitial nephritis with acute renal failure, hepatitis, and meningoencephalitis following systemic Ad infection; she recovered with supportive care.186 There are other cases of meningoencephalitis in which viral isolation from extraneural sites or antibody titer increases have been used to make a diagnosis, especially associated with epidemic Ad7 pneumonia in children.664 A case of sudden unilateral deafness was associated with an Ad3 infection of the nasopharynx.330
Gastrointestinal Diseases
Gastroenteritis, or inflammation of the stomach and small and large intestines, is characterized by fever, vomiting, and diarrhea. It is frequently caused by viruses, bacteria, or parasites. Viruses that cause gastroenteritis include rotaviruses, Ads, noroviruses, calciviruses, astroviruses, and Norwalk virus. Rotaviruses are the leading cause of diarrhea in the world, and calciviruses cause the most gastroenteritis outbreaks in industrial nations.229 The role of Ads is discussed in the next few sections.
Diarrhea
The relationship between Ads and diarrhea has had a long and complicated history but has now been clarified.308,787 Because many Ads replicate efficiently in the intestine and are excreted in the stool, it was assumed that they would be strong candidates for causing diarrhea. However, most earlier epidemiologic studies generally found as many Ad isolates in the stools of controls as in those with diarrhea.578 The failure to correlate Ad growth from stool with clinical illness was a good example that Ads should not be designated as the cause of a whole spectrum of medical illnesses just because they can be cultured from the stool of an individual with a disease. Asymptomatic children can clearly shed Ads in stool and often develop antibodies to the particular type grown.205,290 These subclinical infections probably result in lifelong immunity.
The whole issue of Ads as a cause of infantile gastroenteritis was re-examined based on the observation that initially “noncultivatable” Ads were seen on EM examination of stool smears of affected children.201 Serologic detection methods such as ELISA and alternate tissue culture host–cell systems, such as the HEK 293 line, have identified several of these “noncultivatable” viruses.147,781 Two different enteric Ads, Ad40 and 41, have been associated with diarrhea.147,226,781
Epidemiologic studies to assess the importance of these agents have been completed for several population groups. A report of 14 enteric Ad–related cases of diarrhea in 27 hospitalized patients studied during a 12-week period suggested that these viruses may be an important cause of acute gastrointestinal disease in hospitalized young children and may be nosocomially transmitted; this report also suggested that respiratory symptoms may be a prominent part of the clinical manifestations.802 The incidence of Ad-related gastroenteritis differs considerably in the various studies and locations reported by many authors. In general, it is not as prevalent as rotavirus diarrhea, occurs most often in children younger than 4 years of age, and is not easily distinguished on clinical grounds from rotavirus
infection.587 In Bangladesh, it was responsible for 2.8% of the cases of diarrhea but reached 12.3% in some months as diagnosed by a monoclonal antibody specific for Ad40 and Ad41.333 In day care centers in Houston, 38% of 249 children present during 10 separate outbreaks had diarrhea associated with enteric Ads. Of these patients, 46% were asymptomatic, demonstrating that even during epidemics of enteric Ads, many infected children do not develop gastroenteritis.745 In another study, the prevalence of all Ads in stool was 8%, and Ad40 and Ad41 were 2% in both 565 patients with diarrhea and 129 controls.420 In a recent study of 44 infants in a day care center in Tokyo, Japan, Ad was found in 12.5% of fecal specimens from symptomatic and 11.5% from asymptomatic infants; one outbreak of acute gastroenteritis in these infants was linked to Ad12.6 In another recent study using ELISA analysis of 3,577 fecal specimens from infants and children with acute gastroenteritis in Japan, Korea, and Vietnam during 1998 and 2001, 4.4% were positive for Ad.424 More than half of these were Ad41, but Ad40, 2, 3, 8, and 31 were also detected. Enteric Ads were detected in a fraction of stool samples of children with gastroenteritis in a number of countries, as follows: 6.9% in the United States72; 8.0% in Sweden735; 8.3% in Germany538; 7.9% in the East Anglia, United Kingdom665; 9% in Belfast, United Kingdom534; 1.4% in Blantyre, Malawi135; 1.5% in Brazil673; 6.7% in Iran619; 4% in Jakarta, Indonesia690; 10.8% in Shenzhen, China274; and 4% in Jakarta, Indonesia.690 In many of these studies, nonenteric Ads were also detected but at lower frequencies. In Japan and Iran, antibodies to enteric Ad were detected in sera from about half of healthy children analyzed.618,659 Thus, it is clear that the role of enteric Ads as a cause of diarrhea is roughly the same in different areas of the world.
infection.587 In Bangladesh, it was responsible for 2.8% of the cases of diarrhea but reached 12.3% in some months as diagnosed by a monoclonal antibody specific for Ad40 and Ad41.333 In day care centers in Houston, 38% of 249 children present during 10 separate outbreaks had diarrhea associated with enteric Ads. Of these patients, 46% were asymptomatic, demonstrating that even during epidemics of enteric Ads, many infected children do not develop gastroenteritis.745 In another study, the prevalence of all Ads in stool was 8%, and Ad40 and Ad41 were 2% in both 565 patients with diarrhea and 129 controls.420 In a recent study of 44 infants in a day care center in Tokyo, Japan, Ad was found in 12.5% of fecal specimens from symptomatic and 11.5% from asymptomatic infants; one outbreak of acute gastroenteritis in these infants was linked to Ad12.6 In another recent study using ELISA analysis of 3,577 fecal specimens from infants and children with acute gastroenteritis in Japan, Korea, and Vietnam during 1998 and 2001, 4.4% were positive for Ad.424 More than half of these were Ad41, but Ad40, 2, 3, 8, and 31 were also detected. Enteric Ads were detected in a fraction of stool samples of children with gastroenteritis in a number of countries, as follows: 6.9% in the United States72; 8.0% in Sweden735; 8.3% in Germany538; 7.9% in the East Anglia, United Kingdom665; 9% in Belfast, United Kingdom534; 1.4% in Blantyre, Malawi135; 1.5% in Brazil673; 6.7% in Iran619; 4% in Jakarta, Indonesia690; 10.8% in Shenzhen, China274; and 4% in Jakarta, Indonesia.690 In many of these studies, nonenteric Ads were also detected but at lower frequencies. In Japan and Iran, antibodies to enteric Ad were detected in sera from about half of healthy children analyzed.618,659 Thus, it is clear that the role of enteric Ads as a cause of diarrhea is roughly the same in different areas of the world.
Intussusception
Another intestinal syndrome, intussusception, has been linked in some patients to Ad infection.45,687 The telescoping bowel characteristic of intussusception may be caused by mesenteric adenitis acting as a lead point to the mechanical obstruction. Ads (species C, HA group III, Ad1, 2, 5, and 6) have been isolated from both stool cultures and the involved lymph nodes removed at surgery.122,571 The percentage of children with intussusception showing evidence of Ad is high as evidenced by shedding into stool, detection in throat swabs, anti-Ad antibodies, Ad inclusion bodies in tissue samples, and identification of Ad by election microscopy. The percentages found in a number of studies range from 22% to 61% (see references in308). Most of the studies examined control patients, and in all cases the presence of Ad was statistically more significant than in the controls. These studies were from several parts of the world (United States, United Kingdom, Spain, France, Taiwan, Nigeria). In an analysis using immunohistochemistry and PCR of formalin-fixed intestinal specimens from 12 Mexican pediatric patients, 4 patients (33%) were positive for species C Ad.251 There is no evidence that Ad40 and Ad41 are involved in this syndrome.59 A proposed alternative explanation is that some hyperirritability of the small intestine might be caused by Ad infection and lead to the intussusception.808 However, many patients with intussusception have no evidence of Ad infection, and the disease is probably multifactorial. Ad inclusions have been seen in about one-third to one-half of appendices removed at surgery.308
Celiac Disease
Celiac disease is a common autoimmune disease thought to be caused by ingesting the proteins of the gliadin, hordein, and secalin classes found in wheat, barley, and rye. Structural homology between the Ad12 E1B-55K protein and A-gliadin, a major component of gluten proteins known to activate celiac disease, was noted. In addition, most patients with celiac disease had evidence of prior Ad12 infection, in contrast to matched controls. These observations raise the possibility that the E1B-55K protein from an Ad found in the intestine may play a role in the pathogenesis of celiac disease, perhaps by inducing cross-reacting antibodies to A-gliadin.346 In support of this possibility, T lymphocytes from celiac patients recognize a synthetic dodecapeptide shared by the Ad12 E1B-55K protein and A-gliadin.456 On the other hand, in three studies using PCR to determine whether there was persistent Ad12 infection in the intestinal mucosa of patients with celiac disease, 4 of 18 patients had detectable Ad12 DNA, as did 2 of 24 controls, leading the authors to conclude that Ad12 persistence is not a major element in celiac disease.451 Similar conclusions were reached by others but do not preclude that prior Ad12 infection might have been involved in the evolution of celiac disease.407,753
Myocarditis
Myocarditis is an inflammatory disorder of the myocardium characterized by necrosis of myocytes and infiltration of inflammatory cells. The most common form of this disease, viral myocarditis, is generally thought to be caused by enterovirus infection (e.g., coxsackievirus), although there is evidence that Ad may be a significant cause as well (reviewed in69,356,453). Ads do not normally cause symptomatic infection of the heart, and the reason that it is associated with myocarditis and dilated cardiopathy is unknown. Both enteroviruses and Ads can infect cardiac myocytes in culture. It is of interest that coxsackieviruses and Ads both use CAR as the receptor to enter cells. CAR expression in healthy and diseased hearts is reported to be variable, and it has been suggested that this variability plays a role in enterovirus and Ad myocarditis.570
A number of reports have diagnosed Ad in myocarditis and dilated myocardiopathy. In a study of 126 conscripts diagnosed on the basis of serial electrocardiograph changes during an acute infection, Ad-specific antibodies increased in 19 patients, although most did not have myocarditis.362 Ad DNA has been detected by PCR in myocardial biopsies primarily from children with acute myocarditis in three studies (see308). Although there was clinical and electrocardiogram evidence of myocarditis, most of the Ad-positive biopsies did not show classic inflammation, in contrast to those that appeared to be associated with enterovirus infection. In children with myocarditis or dilated cardiomyopathy, Ad was detected in anywhere from 8.1% to 36% of patients.67,68,83,397,621,626,657 Sequencing of the PCR products from one of these studies established that 80% of the Ad isolates were Ad2; most of the remainder were Ad5 and one was Ad6; nonfatal cases caused by Ad1 have also been described.621 In another study of 142 Ad-positive patients with clinical diagnosis of myocarditis, 57 (40%) had histopathology that was consistent with myocarditis; none of the control patients were positive for Ad.67 Some patients with detected virus remain positive for a prolonged period of time (mean of 6.8 months); clearance of the virus was associated with improved symptoms.397 Myocardial involvement with Ad may
be patchy, suggesting that biopsies may miss affected regions and thereby underestimate the incidence of Ad-associated disease.268 These studies suggest that Ad may be a cause of or a contributing factor for cardiomyopathy.
be patchy, suggesting that biopsies may miss affected regions and thereby underestimate the incidence of Ad-associated disease.268 These studies suggest that Ad may be a cause of or a contributing factor for cardiomyopathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree