, Arthur H. Cohen2, Robert B. Colvin3, J. Charles Jennette4 and Charles E. Alpers5
(1)
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
(2)
Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA
(3)
Department of Pathology Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA
(4)
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
(5)
Department of Pathology, University of Washington, Seattle, Washington, USA
Abstract
Acute tubular necrosis (ATN) is a pathologic process that manifests clinically as acute renal failure. Although the term implies cellular death (necrosis), it should be appreciated that frank necrosis is not a constant finding; evidence of sublethal cellular injury is common. Thus, a more descriptive term “acute tubular injury” is in common use in many centers. Furthermore, there is often a lack of clinical-pathologic correlation, with severe acute kidney injury sometimes associated with trivial morphologic findings [1, 2]
Introduction/Clinical Setting
Acute tubular necrosis (ATN) is a pathologic process that manifests clinically as acute renal failure. Although the term implies cellular death (necrosis), it should be appreciated that frank necrosis is not a constant finding; evidence of sublethal cellular injury is common. Thus, a more descriptive term “acute tubular injury” is in common use in many centers. Furthermore, there is often a lack of clinical-pathologic correlation, with severe acute kidney injury sometimes associated with trivial morphologic findings [1, 2]
Broadly speaking, ATN may be the result of one of two mechanisms: ischemia or toxin induced. The structural changes in each are reasonably distinctive, and pathogenic mechanisms are also considered different. Traditionally, ischemic ATN follows hypotension or hypovolemia or both [1, 3]. There may be many causes of this circulatory state; these include extensive trauma with rhabdomyolysis and myoglobinuria, incompatible blood transfusions, pancreatitis, septic shock in a variety of settings, extensive hemolysis as in malaria (blackwater fever), and shock following administration of barbiturates, morphine, and sedatives. Toxic ATN is a dose-dependent injury with tubular cell damage normally limited to proximal tubules and usually involving almost all nephrons. This is obviously in sharp contrast to ischemic tubular necrosis in which the changes are considerably more subtle and patchy. Many therapeutic and diagnostic agents, industrial chemicals, heavy metals, and plants may be responsible for this lesion.
Ischemic Acute Tubular Necrosis
Pathologic Findings
The pathologic changes in ischemic ATN are often subtle but are easily discernible with well-fixed tissue. Both proximal and distal tubules are affected. The proximal tubules are dilated and the lining cells flattened. Brush border staining is reduced or absent (Fig. 15.1). This combination of changes causes proximal tubules to resemble distal tubules (“distalization”) and is also known as simplification of tubules so that one nephron segment cannot easily be differentiated from another. Distal tubules, including the thick ascending limb of Henle, may be dilated and lined by flattened cells. Tamm-Horsfall protein-containing casts, sometimes incorporating granular material, are frequently present but are not specific for ATN. In addition, pigmented (tan-brown) granular casts are characteristic; many studies have concluded that heme pigment is responsible for this appearance. Oxalate crystals are commonly present and are located in the thick ascending limb, distal tubule, and collecting duct. It should be noted that the crystals are usually not numerous; marked accumulation of oxalate crystals is most commonly observed in ethylene glycol poisoning. Overt or extensive necrosis of tubular cells is neither common nor regularly observed. Instead, loss of individual cells, manifested by incomplete epithelial lining of tubules, is present. This change requires well-fixed tissue and a practiced eye to demonstrate. It is often referred to as the “nonreplacement” phenomenon. There are often desquamated cells or cellular debris in the lumina (Fig. 15.2).
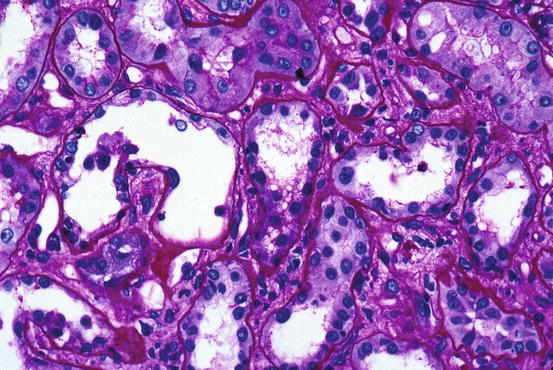
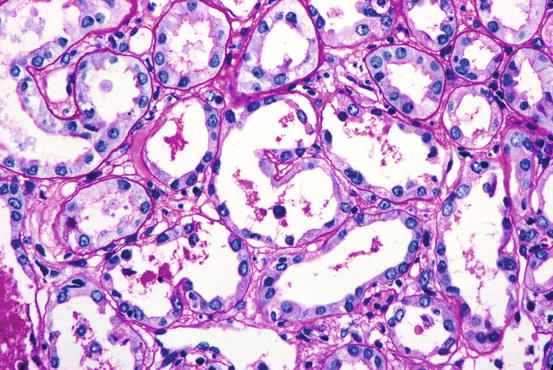

Fig. 15.1
Tubular cells are flattened and many proximal cells lack brush border staining; lumina are relatively dilated [periodic acid-Schiff (PAS) stain]

Fig. 15.2
One tubule contains cells and debris in lumen and is incompletely lined by epithelium (PAS stain)
Ischemic tubular necrosis is frequently associated with disruption of tubular walls (including cell loss and basement membrane disruption) with spillage of contents into the adjacent interstitium and rarely into peritubular capillaries and small veins. It also may be associated with localized inflammation, sometimes in the form of granulomata. However, inflammation is not constantly present. The interstitium is diffusely edematous and may be infiltrated by a small number of lymphocytes and monocytes. The outer medullary vasa recta often contain large numbers of nucleated circulating cells including lymphocytes and monocytes, both mature and immature forms, and granulocyte precursors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


