INTRODUCTION
LEARNING OBJECTIVES
After studying this chapter you should:
Understand the pathogenesis and treatment of vitamin K deficiency.
Understand the pathogenesis, diagnosis, and treatment of disseminated intravascular coagulation.
One of the most challenging aspects of clinical medicine is the diagnosis and treatment of acquired bleeding disorders. These problems commonly arise in hospitalized patients on medical, surgical, and obstetrical services. The immediate question of whether the bleeding is due to recent trauma or surgery is sometimes difficult to answer with certainty. A careful history may reveal an inherited bleeding disorder or exposure to drugs or toxins that might adversely affect platelet or coagulation functions. A thorough physical examination will usually reveal whether the bleeding is local and restricted to the area of trauma or surgery, rather than widespread. In any case, a patient with unexplained hemorrhage, local or systemic, should be initially evaluated and then closely monitored with baseline screening tests that include a platelet count, prothrombin time, and partial thromboplastin time. Occasionally, additional coagulation tests are needed to delineate the cause of the bleeding and to guide appropriate therapy. Skillful management of these patients, who often have complex illnesses, must be based on a solid understanding of the pathophysiology of hemostasis, as presented in Chapter 13 and amplified in this chapter.
In contrast to inherited conditions such as hemophilia (Chapter 15), acquired bleeding disorders usually involve deficiencies of multiple clotting factors. This chapter will focus primarily on three problems commonly encountered on medical and surgical floors: vitamin K deficiency, severe liver disease, and disseminated intravascular coagulation (DIC).
VITAMIN K DEFICIENCY
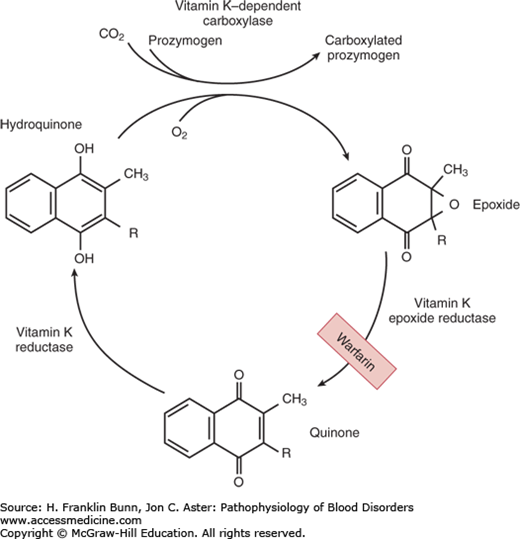
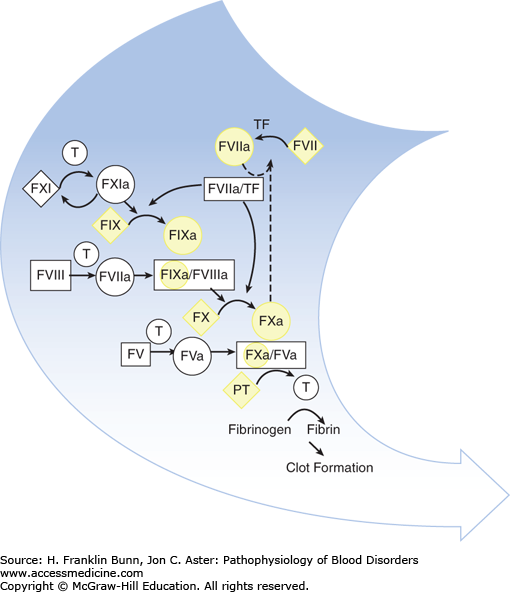
Vitamin K is a fat-soluble organic compound found primarily in leafy green vegetables but also in meat and dairy products. It is composed of a bicyclical naphthoquinone backbone and an aliphatic side chain. As shown in Figure 16-1, vitamin K is converted to the hydroquinone by a vitamin K reductase. The hydroquinone is an essential cofactor utilized by the vitamin K–dependent carboxylase to catalyze the oxidative fixation of carbon dioxide onto specific glutamic acid residues to form γ-carboxyglutamic acid. During this reaction vitamin K is converted to an epoxide, which is then converted back to the quinone by a vitamin K epoxide reductase. This vitamin K–dependent post-translational modification takes place in the Golgi apparatus of many cell types and is restricted to specific domains on a small number of proteins—those known as vitamin K–dependent proteins. In hepatocytes, γ-carboxylation is necessary for the biologic activity of the vitamin K– dependent coagulation factors: the zymogens prothrombin, factors VII, IX, and X (Fig. 16-2), as well as protein C and protein S. In bone, γ-carboxylation is necessary for the synthesis of osteocalcin and matrix Gla protein. In all cases, this structural modification enables binding of divalent calcium. The function of vitamin K– dependent clotting factors requires calcium binding in order to form procoagulant complexes on membrane surfaces.
FIGURE 16-1
The vitamin K cycle. This figure shows the enzymatic steps that convert vitamin K to the hydroquinone required for participation as a cofactor in γ-carboxylation of glutamic acid residues, and the regeneration of vitamin K by conversion of the epoxide back to the quinone. (Modified with permission from Furie BC and Furie B. Vitamin K metabolism and disorders. In Hoffman R, Benz EJ, Shattil SJ, et al, eds. Hematology: Basic Principles and Practice, 3rd Edition. New York, USA, Churchill Livingstone, 2001:1959.)
FIGURE 16-2
The participation of vitamin K–dependent clotting factors highlighted in yellow in the coagulation cascade. (Modified with permission from Furie B and Furie BC. The molecular basis of blood coagulation. In Hoffman R, Benz EJ, Shattil SJ, et al, eds. Hematology, Basic Principles and Practice, 3rd Edition, New York, USA, Churchill Livingstone, 2001:1784.)
Vitamin K is absorbed in the intestine. Sources of vitamin K include food and vitamin K synthesized by flora in the gut. Accordingly, patients who are not eating and who are receiving broad-spectrum antibiotics are at risk for becoming vitamin K deficient. This scenario is commonly encountered, particularly in hospitalized patients, and is often not diagnosed until they develop clinically significant bleeding. Less often, vitamin K deficiency is caused by obstructive liver disease or by disorders of the intestinal mucosa that lead to chronic malabsorption.
Formerly, vitamin K deficiency in newborns sometimes caused ecchymoses, bleeding from venipuncture sites, and, rarely, intracranial hemorrhage. Hemorrhagic disease of the newborn arises because plasma levels of vitamin K–dependent clotting factors are relatively low at birth, the gastrointestinal tract of the newborn is relatively sterile, and mother’s milk contains very little vitamin K. The risk is enhanced if the mother has been taking antibiotics or anticonvulsant drugs. Fortunately, this complication is now rarely encountered, owing to the routine administration of vitamin K to newborns.
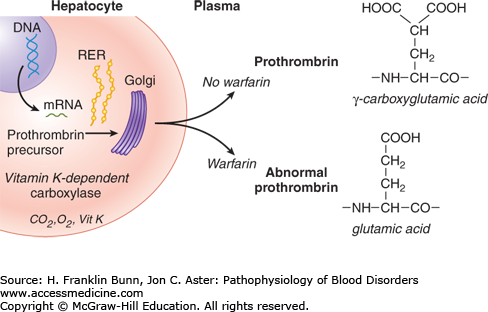
An important and common cause of functional “vitamin K deficiency” is overdosage with anticoagulant drugs, primarily warfarin. These drugs are competitive inhibitors of vitamin K epoxide reductase (Fig. 16-1). As shown in Figure 16-3, warfarin administration results in a decrease in γ-carboxylation of prothrombin and other vitamin K–dependent clotting factors. Patients taking warfarin are carefully monitored through regular measurement of the prothrombin time, which allows the dose of warfarin to be adjusted to maintain the patient within a safe and effective anticoagulant range. Patients are at risk of abnormal bleeding when the prothrombin time significantly exceeds this range. Occasionally, mentally disturbed patients develop hemorrhage after self-administration of toxic doses of warfarin or another vitamin K antagonist such as rodent poison.
FIGURE 16-3
The biosynthesis of proteins modified by γ-carboxylation of glutamic acid residues and the inhibition of this post-translational modification by warfarin. (Modified with permission from Flaumenhaft R and Furie B. Biochemistry of factor IX and molecular biology of hemophilia B. In Hoffman R, Benz EJ, Shattil SJ, et al, eds. Hematology: Basic Principles and Practice, 3rd Edition. New York, USA, Churchill Livingstone, 2001:1870.)