Chapter 8 Acid-Base Balance
I. Acid and Base
A. Overview
1. The pH (i.e., −log [H+]) of the body is maintained within a very narrow range to allow for proper protein functioning.
2. Normal plasma [H+] is therefore very low at approximately 40 nmol; Table 8-1 provides a comparison to that of other plasma ions.
3. Such tight control is due to the presence of buffers in all of the body compartments, removal of volatile acids by the lungs, and excretion of nonvolatile acids by the kidneys.
TABLE 8-1 Normal Concentrations of Cations and Anions in Plasma
| Cations (mEq/L) | Anions (mEq/L) |
|---|---|
| Na+ 140 | Cl– 103 |
| K+ 4 | 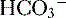 25 25 |
| Ca2+ 5 (2.5 mmol/l) | Proteins 16 |
| Mg2+ 2 (1 mmol/L) | Organic 4 |
| H+ 0.000040 (40 nmol/L) | Other inorganics 3 |
From Halperin M, Goldstein M: Fluid, Electrolyte, and Acid-Base Physiology, 2nd ed. Philadelphia, Saunders, 1994, Table 1-2.
4. Daily metabolism of fats and carbohydrates to CO2 produces a substantial volatile acid load in the form of CO2 (approximately 15,000 mmol), which forms H+ ions through the following reaction: H2O + CO2 → H2CO3 → H+ + 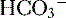 .
.
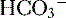 .
.5. Daily metabolism of proteins produces a much smaller acid load (approximately 50 to 100 mEq) in the form of nonvolatile acids such as sulfate and phosphate.
6. The primary buffer of the extracellular fluid (ECF) compartment is bicarbonate, which buffers the daily load of acid generated by metabolism.
7. The lungs contribute to acid-base balance by excreting CO2, although in acid-base disorders (e.g., metabolic alkalosis), they can “compensate” by retaining CO2.
8. The kidneys contribute to acid-base balance by removing nonvolatile acids such as sulfate and phosphate; they also reclaim most of the filtered bicarbonate and create de novo bicarbonate through deamination of the amino acid glutamine.
9. Diagnosis of an acid-base disorder can often be made by the presence of electrolyte abnormalities alone with a suggestive history (e.g., high [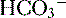 ], low [Cl-] after vomiting suggests a metabolic alkalosis).
], low [Cl-] after vomiting suggests a metabolic alkalosis).
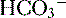 ], low [Cl-] after vomiting suggests a metabolic alkalosis).
], low [Cl-] after vomiting suggests a metabolic alkalosis).10. A low plasma pH is referred to as an acidemia, whereas a process resulting in the production of excess acids is termed an acidosis, irrespective of the pH.
B. Acids, bases, and buffers
1. Acids are compounds that can donate a hydrogen ion, whereas bases are compounds that can accept a hydrogen ion.
2. Assuming the reaction HA → H+ + A−, the higher the concentration of a conjugate base (A−) relative to its acidic form (HA), the higher will be the pH.
3. This relationship is demonstrated by the Henderson-Hasselbalch equation, shown below.
which can be rewritten as:
4. Note that the pKa equals the pH at which the acid is half dissociated; in other words, the pH at which [A] = [HA].
6. In this system, carbonic acid (H2CO3), a weak acid, rapidly dissociates into either CO2 or 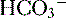 , as shown here:
, as shown here:
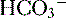 , as shown here:
, as shown here: C. Role of the kidneys in acid-base balance
1. Overview
• The kidneys filter approximately 4500 mEq of bicarbonate daily (24 mEq/L × 180 L/day); most of this is reclaimed.
2. Bicarbonate reclamation
• More than 99.9% of filtered plasma bicarbonate is reabsorbed in the kidney: approximately 80% in the proximal tubule, 10% in the thick ascending limb, and 10% in the distal nephron.
• Hydrogen ions are secreted into the tubular lumen, where they react with filtered bicarbonate to form carbonic acid, which dissociates into CO2 and water in a reaction catalyzed by carbonic anhydrase.
• The CO2 and water diffuse across the tubular cell membrane, and inside the cell, the reverse reaction takes place.
• The resulting bicarbonate is pumped out of the basolateral surface of the cell and returned to the plasma.
3. De novo bicarbonate synthesis
• The removal of a hydrogen ion is the biochemical equivalent of bicarbonate generation, so the kidney needs to excrete hydrogen ions to generate bicarbonate and to prevent acidosis.
• If H+ were excreted as free ions in the urine, this would lower urine pH to physiologically intolerable levels; in fact, negligible amounts of free H+ ions are excreted because the kidney uses urinary buffers to facilitate H+ excretion.
• The buffers used are filtered weak acids that make up what is called titratable acidity and ammonium; using bicarbonate as a buffer would accomplish nothing, because the effect of losing H+ would be offset by the simultaneous loss of 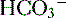 .
.
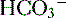 .
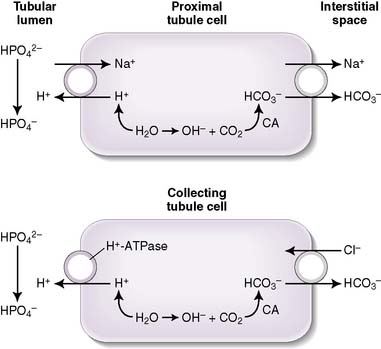
.a. Titratable acidity
• Filtered phosphate (HPO4−2) is the major contributor to titratable acidity (Fig. 8-2); less abundant acids with less favorable pKa values (e.g., creatinine and uric acid) also contribute.
• The amount of titratable acidity present in the urine can be determined by determining the amount of OH required to titrate the urine pH back to 7.4; hence, the name titratable acidity.
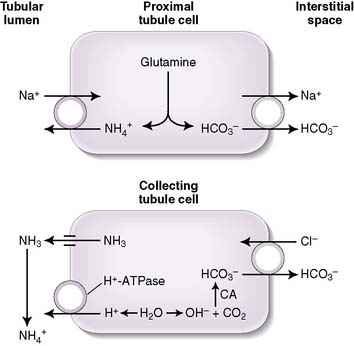
b. Ammonium production
• The deamination of the amino acid glutamine in the proximal tubule cells yields two ammonium (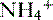 ) molecules and two bicarbonate molecules (Fig. 8-3).
) molecules and two bicarbonate molecules (Fig. 8-3).
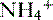 ) molecules and two bicarbonate molecules (Fig. 8-3).
) molecules and two bicarbonate molecules (Fig. 8-3).• The bicarbonate molecules are transported across the basolateral membrane and diffuse into the peritubular capillaries.
• The 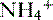 is transported across the luminal membrane by substitution of
is transported across the luminal membrane by substitution of 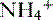 on the Na+-H+ countertransporter.
on the Na+-H+ countertransporter.
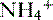 is transported across the luminal membrane by substitution of
is transported across the luminal membrane by substitution of 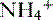 on the Na+-H+ countertransporter.
on the Na+-H+ countertransporter.c. Regulation of de novo bicarbonate synthesis
• Under normal physiologic circumstances, all of the filtered bicarbonate is reabsorbed, and the additional amount of bicarbonate required to offset the 40 to 80 mEq of H+ produced daily is generated in the kidney by excretion of titratable acids and ammonium.
• Renal acid excretion, and hence bicarbonate synthesis, varies to adapt to different physiologic circumstances:
• The amount of H+ excreted varies inversely with extracellular pH; as systemic pH falls, the activities of the kidney’s Na+-H+ countertransporter, H+-ATPase cotransporter, and Na+–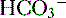 cotransporter increase.
cotransporter increase.
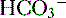 cotransporter increase.
cotransporter increase.• The capacity of titratable acidity to increase is fairly limited, so the required increase in renal buffering capacity is derived from increased production of 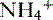 .
.
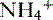 .
.• Systemic alkalosis results in a reversal of these H+-secreting processes and a decrease in bicarbonate reabsorption.
D. Metabolic acidosis
1. Overview




• Metabolic acidoses can be divided into anion gap and normal anion gap acidoses. See Box 8-1 for a clinical example of normal anion gap metabolic acidosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


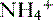 ) and titratable acids.
) and titratable acids.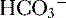 ] above could represent metabolic compensation for a respiratory acidosis.
] above could represent metabolic compensation for a respiratory acidosis.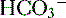 ), its (conjugate) base.
), its (conjugate) base.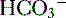 : its conjugate base
: its conjugate base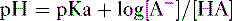
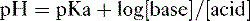
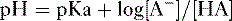
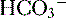 /CO2 system most important buffer in the plasma
/CO2 system most important buffer in the plasma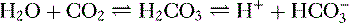
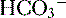 ) are able to be rapidly excreted by the lungs and kidneys, respectively.
) are able to be rapidly excreted by the lungs and kidneys, respectively.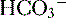 to be rapidly removed by the lungs and kidneys, respectively.
to be rapidly removed by the lungs and kidneys, respectively.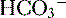 is produced and
is produced and 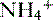 is excreted in urine
is excreted in urine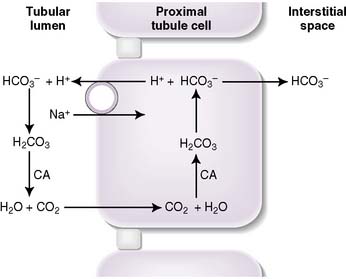
 molecules and two
molecules and two 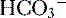 molecules
molecules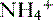 , which is then trapped in the tubular lumen.
, which is then trapped in the tubular lumen.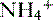 being trapped in the tubular lumen is an oversimplification of renal
being trapped in the tubular lumen is an oversimplification of renal 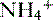 handling. In fact,
handling. In fact, 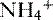 is produced, partially reabsorbed, and then dissociated to NH3, which is recycled in the renal medulla, where its high concentration prompts diffusion back into the tubular lumen; there, it combines again with secreted H+ to form
is produced, partially reabsorbed, and then dissociated to NH3, which is recycled in the renal medulla, where its high concentration prompts diffusion back into the tubular lumen; there, it combines again with secreted H+ to form 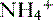 . The net result is that
. The net result is that 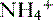 ends up back in the tubular lumen.
ends up back in the tubular lumen.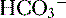 reclamation: normally approximates 100%
reclamation: normally approximates 100%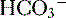 (<22 mEq/L) and low P
(<22 mEq/L) and low P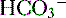 ], low P
], low P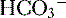 can also be seen in compensation for a respiratory alkalosis, although in this case the pH would be high rather than low.
can also be seen in compensation for a respiratory alkalosis, although in this case the pH would be high rather than low.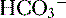 16
16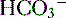 -rich intestinal fluid, causing a hyperchloremic metabolic acidosis. Note that this is a normal anion gap metabolic acidosis because there is no production of an unmeasured anion. Based on Winter’s formula, appropriate respiratory compensation (through hyperventilation) should result in a drop in P
-rich intestinal fluid, causing a hyperchloremic metabolic acidosis. Note that this is a normal anion gap metabolic acidosis because there is no production of an unmeasured anion. Based on Winter’s formula, appropriate respiratory compensation (through hyperventilation) should result in a drop in P