Objectives
- Defines acids, bases, and buffers.
- Lists the buffer systems available in the human body.
- Describes the interrelationships of the pH, the
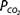 of the blood, and the plasma bicarbonate concentration, and states the Henderson-Hasselbalch equation.
of the blood, and the plasma bicarbonate concentration, and states the Henderson-Hasselbalch equation.
- States the normal ranges of arterial pH,
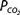 , and bicarbonate concentration, and defines alkalosis and acidosis.
, and bicarbonate concentration, and defines alkalosis and acidosis.
- Lists the potential causes of respiratory acidosis and alkalosis and metabolic acidosis and alkalosis.
- Discusses the respiratory and renal mechanisms that help compensate for acidosis and alkalosis.
- Evaluates blood gas data to determine a subject’s acid-base status.
- Classifies and explains the causes of tissue hypoxia.
Acid-Base Balance: Introduction
The maintenance of a relatively constant internal environment is one of the major physiologic functions of the organ systems of the body. Body temperature, fluid volume and osmolarity, and electrolytes—including acids and bases—are normally carefully regulated. A thorough knowledge of the mechanisms that control these variables is essential to clinical practice.
The respiratory system is intimately involved in the maintenance of the balance of acids and bases in the body. This chapter will introduce the major concepts of acid-base balance, particularly with respect to the respiratory system; a more detailed study of this important subject is strongly encouraged.
The Chemistry of Acids, Bases, and Buffers
Although there are several ways to define acids and bases, the most useful physiologically is to define an acid as a substance that can donate a hydrogen ion (a proton) to another substance and a base as a substance that can accept a hydrogen ion from another substance. A strong acid is a substance that is completely or almost completely dissociated into a hydrogen ion and its corresponding or conjugate base in dilute aqueous solution; a weak acid is only slightly ionized in aqueous solution. In general, a strong acid has a weak conjugate base and a weak acid has a strong conjugate base. The strength of an acid or a base should not be confused with its concentration.
A buffer is a mixture of substances in aqueous solution (usually a combination of a weak acid and its conjugate base) that can resist changes in hydrogen ion concentration when strong acids or bases are added. That is, the changes in hydrogen ion concentration that occur when a strong acid or base is added to a buffer system are much smaller than those that would occur if the same amount of acid or base were added to pure water or another nonbuffer solution.
The acidity of a solution is determined by the activity of the hydrogen ions in the solution. The hydrogen ion activity, which is denoted by the symbol αH+, is closely related to the concentration of hydrogen ions ([H+]) in a solution. In extremely dilute solutions, the hydrogen ion activity is equal to the hydrogen ion concentration; in highly concentrated solutions, the activity is less than the concentration. The hydrogen ion concentration of the blood is low enough that the hydrogen ion activity may be considered to be equal to the hydrogen ion concentration.
The hydrogen ion activity of pure water is about 1.0 × 10−7 mol/L. By convention, solutions with hydrogen ion activities above 10−7 mol/L are considered to be acid; those with hydrogen ion activities below 10−7 are considered to be alkaline. The range of hydrogen ion concentrations or activities in the body is normally from about 10−1 for gastric acid to about 10−8 for the most alkaline pancreatic secretion. This wide range of hydrogen ion activities led to the use of the somewhat more convenient pH scale. The pH of a solution is the negative logarithm of its hydrogen ion activity. With the exception of the highly concentrated gastric acid, in most instances in the body the hydrogen ion activity is about equal to the hydrogen ion concentration. Therefore,
pH = −log (αH +)
or pH = −log [H+]
Thus, the pH of gastric acid is on the order of 1; the pH of the alkaline pancreatic secretion may be as high as 8.
The pH of arterial blood is normally close to 7.40, with a normal range considered to be about 7.35 to 7.45. An arterial pH less than 7.35 is considered acidemia; an arterial pH greater than 7.45 is considered alkalemia. The underlying condition characterized by hydrogen ion retention or by loss of bicarbonate or other bases is referred to as acidosis; the underlying condition characterized by hydrogen ion loss or retention of base is referred to as alkalosis. Under pathologic conditions the extremes of arterial blood pH have been noted to range as high as 7.8 and as low as 6.9. These correspond to hydrogen ion concentrations as follows [hydrogen ion concentrations are expressed as nanomoles (10−9 mol/L) for convenience]:
| pH | Concentration (nmol/L) |
|---|---|
| 6.90 | 126 |
| 7.00 | 100 |
| 7.10 | 79 |
| 7.20 | 63 |
| 7.30 | 50 |
| 7.40 | 40 |
| 7.50 | 32 |
| 7.60 | 25 |
| 7.70 | 20 |
| 7.80 | 16 |
Note that the pH scale is “inverted” by the negative sign and is also logarithmic. Therefore, an increase in pH from 7.40 to 7.70 represents a decrease in hydrogen ion concentration. The increase of only 0.3 pH units indicates that hydrogen ion concentration is cut in half because the logarithm of 2 in base 10 is 0.3.
Hydrogen ions are the most reactive cations in body fluids, and they interact with negatively charged regions of other molecules, such as those of body proteins. Interactions of hydrogen ions with negatively charged functional groups of proteins can lead to marked changes in protein structural conformations with resulting alterations in the behavior of the proteins. An example of this was already seen in Chapter 7, where hemoglobin was noted to combine with less oxygen at a lower pH (the Bohr effect). Alterations in the structural conformations and charges of protein enzymes can affect their activities, with resulting alterations in the functions of body tissues. The absorption and efficacy of drugs administered by the physician may also be affected by the pH. Extreme changes in the hydrogen ion concentration of the body can result in loss of organ system function and structural integrity; under acute conditions, arterial pHs above approximately 7.80 or below 6.9 are not compatible with life.
Under normal circumstances, cellular metabolism is the main source of acids in the body. These acids are the waste products of substances ingested as foodstuffs. The greatest source of hydrogen ions is the carbon dioxide produced as one of the end products of the oxidation of glucose and fatty acids during aerobic metabolism. The hydration of carbon dioxide results in the formation of a hydrogen ion and a bicarbonate ion, as discussed in Chapter 7. This is reversed in the pulmonary capillaries, and CO2 then diffuses through the alveolar-capillary barrier into the alveoli, from which it is removed by alveolar ventilation. Carbonic acid is therefore said to be a volatile acid because it can be converted into a gas and then removed from an open system like the body. Very great amounts of carbon dioxide can be removed from the lungs by alveolar ventilation: Under normal circumstances, about 15,000 to 25,000 mmol of carbon dioxide is removed via the lungs daily.
A much smaller quantity of fixed or nonvolatile acids is also normally produced during the course of the metabolism of foodstuffs. The fixed acids produced by the body include sulfuric acid, which originates from the oxidation of sulfur-containing amino acids such as cysteine; phosphoric acid from the oxidation of phospholipids and phosphoproteins; hydrochloric acid, which is produced during the conversion of ingested ammonium chloride to urea and by other reactions; and lactic acid from the anaerobic metabolism of glucose. Lactic acid is sometimes converted to carbon dioxide, and so it is not always a fixed acid. Other fixed acids may be ingested accidentally or formed in abnormally large quantities by disease processes, such as the acetoacetic and butyric acid formed during diabetic ketoacidosis. About 70 mEq of fixed acids is normally produced and removed from the body each day (about 1 mEq/kg/body weight/day); the range is 50 to 100 mEq. A vegetarian diet may produce significantly less fixed acid and may even result in no net production of fixed acids. The removal of fixed acids is accomplished mainly by the kidneys, as will be discussed later in this chapter. Some may also be removed via the gastrointestinal tract. Fixed acids normally represent only about 0.2% of the total body acid production.
Buffer Systems of the Human Body
The body contains a variety of substances that can act as buffers in the physiologic pH range. These include bicarbonate, phosphate, and proteins in the blood, the interstitial fluid, and inside cells. One way to express the ability of a substance to act as a buffer is its buffer value. The buffer value of a solution is the amount of hydrogen ions in milliequivalents per liter that can be added to or removed from the solution with a resultant change of 1 pH unit. Another way is to determine the substance’s titration curve.
The pK of the acid is another important consideration. An acid, HA, can dissociate into a hydrogen ion, H+, and its base, A−:
According to the law of mass action, the relationship between the undissociated acid and the proton and the base at equilibrium can be expressed as the following ratio:
That is, the product of the concentrations of hydrogen ion and base divided by the concentration of the acid is equal to a constant K, the dissociation constant. This can be rearranged to
The buffer value or buffering capacity of a buffer pair is greatest at or near the pK of the weak acid. Note that when the concentrations of HA and A− are equal, the pH of a solution is equal to its pK.
As already stated, the human body contains a number of buffers and buffer pairs. The isohydric principle states that all the buffer pairs in a homogeneous solution are in equilibrium with the same hydrogen ion concentration. For this reason, all the buffer pairs in the plasma behave similarly, with the relative concentrations of their undissociated acids and their bases determined by their respective pKs.
An implication of the isohydric principle is that the detailed analysis of a single buffer pair, like the bicarbonate buffer system, can reveal a great deal about the chemistry of all the plasma buffers.
The bicarbonate buffer system consists of the buffer pair of the weak acid, carbonic acid, and its conjugate base, bicarbonate. As already stated, in the body:
The ability of the bicarbonate system to function as a buffer of fixed acids in the body is largely due to the ability of the lungs to remove carbon dioxide from the body. In a closed system bicarbonate would not be nearly as effective.
At a temperature of 37°C about 0.03 mmol of carbon dioxide per mm Hg of 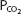 will dissolve in a liter of plasma. (Note that the solubility of CO2 was expressed as milliliters of CO2 per 100 mL of plasma in Chapter 7.) Therefore, the carbon dioxide dissolved in the plasma, expressed as millimoles per liter, is equal to 0.03 ×
will dissolve in a liter of plasma. (Note that the solubility of CO2 was expressed as milliliters of CO2 per 100 mL of plasma in Chapter 7.) Therefore, the carbon dioxide dissolved in the plasma, expressed as millimoles per liter, is equal to 0.03 × 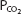 . At body temperature in the plasma, the equilibrium of the second part of the series of equations given above is far to the left so that there is roughly 1000 times as much carbon dioxide present physically dissolved in the plasma as there is in the form of carbonic acid. The dissolved carbon dioxide is in equilibrium with the carbonic acid, though, and so both the dissolved carbon dioxide and the carbonic acid are considered as the undissociated HA in the Henderson–Hasselbalch equation for the bicarbonate system:
. At body temperature in the plasma, the equilibrium of the second part of the series of equations given above is far to the left so that there is roughly 1000 times as much carbon dioxide present physically dissolved in the plasma as there is in the form of carbonic acid. The dissolved carbon dioxide is in equilibrium with the carbonic acid, though, and so both the dissolved carbon dioxide and the carbonic acid are considered as the undissociated HA in the Henderson–Hasselbalch equation for the bicarbonate system:
where [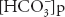 ] stands for plasma bicarbonate concentration. The concentration of carbonic acid is negligible, and so
] stands for plasma bicarbonate concentration. The concentration of carbonic acid is negligible, and so
where pK′ is the pK of the 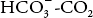 system in blood.
system in blood.
The pK′ of this system at physiologic pHs and at 37°C is 6.1. Therefore, at an arterial pH of 7.40 and an arterial 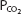 of 40 mm Hg,
of 40 mm Hg,
Therefore, the arterial plasma bicarbonate concentration is about 24 mmol/L (the normal range is 23–28 mmol/L) because the logarithm of 20 is equal to 1.3.
Note that the term total CO2 refers to the dissolved carbon dioxide (including carbonic acid) plus the carbon dioxide present as bicarbonate.
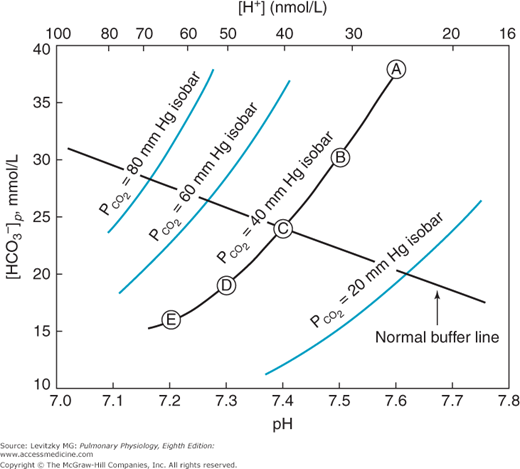
A useful way to display the interrelationships among the variables of pH, 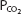 , and bicarbonate concentration of the plasma, as expressed by the Henderson–Hasselbalch equation, is the pH-bicarbonate diagram shown in Figure 8–1.
, and bicarbonate concentration of the plasma, as expressed by the Henderson–Hasselbalch equation, is the pH-bicarbonate diagram shown in Figure 8–1.
Figure 8–1.
The pH-bicarbonate diagram with 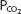 isobars. Note the hydrogen ion concentration in nanomoles per liter at the top of the figure corresponding to the pHs on the abscissa. Points A to E correspond to different pHs and bicarbonate concentrations all falling on the same
isobars. Note the hydrogen ion concentration in nanomoles per liter at the top of the figure corresponding to the pHs on the abscissa. Points A to E correspond to different pHs and bicarbonate concentrations all falling on the same 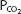 isobar. (Reprinted from Davenport HW. The ABC of Acid-Base Chemistry. 6th ed. 1974, by permission of the University of Chicago Press.)
isobar. (Reprinted from Davenport HW. The ABC of Acid-Base Chemistry. 6th ed. 1974, by permission of the University of Chicago Press.)
As can be seen from Figure 8–1, pH is on the abscissa of the pH-bicarbonate diagram, and the plasma bicarbonate concentration in millimoles per liter is on the ordinate. The hydrogen ion concentration corresponding to the pH on the X-axis is shown at the top of the graph. Note that the hydrogen ion concentration is expressed in nanomoles; the bicarbonate concentration on the Y-axis is expressed in millimoles. For each value of pH and bicarbonate ion concentration, there is a single corresponding 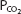 on the graph. Conversely, for any particular pH and
on the graph. Conversely, for any particular pH and 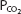 , only 1 bicarbonate ion concentration will satisfy the Henderson–Hasselbalch equation. If the
, only 1 bicarbonate ion concentration will satisfy the Henderson–Hasselbalch equation. If the 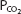 is held constant, for example, at 40 mm Hg, an isobar line can be constructed, connecting the resulting points as the pH is varied. The representative isobars shown in Figure 8–1 give an indication of the potential alterations of acid-base status when alveolar ventilation is increased or decreased. If everything else remains constant, hypoventilation leads to acidosis; hyperventilation leads to alkalosis.
is held constant, for example, at 40 mm Hg, an isobar line can be constructed, connecting the resulting points as the pH is varied. The representative isobars shown in Figure 8–1 give an indication of the potential alterations of acid-base status when alveolar ventilation is increased or decreased. If everything else remains constant, hypoventilation leads to acidosis; hyperventilation leads to alkalosis.
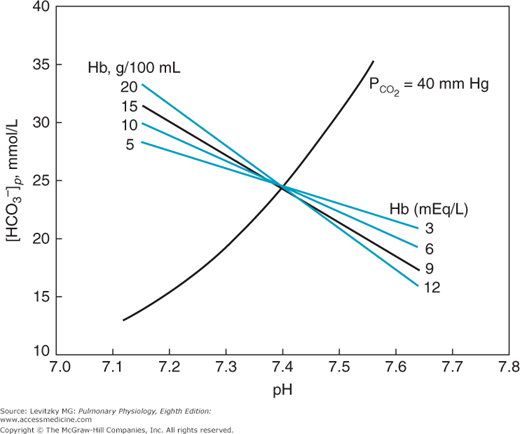
The buffer value of bicarbonate in a closed system without the presence of hemoglobin is only about −5.4 mmol/L/pH unit. That is, the bicarbonate concentration increases only 5.4 mmol/L as enough acid in the form of carbon dioxide is added to the bicarbonate system to lower the pH by 1 unit. The bicarbonate buffer system is therefore a poor buffer for carbonic acid. The presence of hemoglobin makes blood a much better buffer, as can be seen in Figure 8–2.
The figure shows that increasing the hemoglobin concentration in this in vitro experiment makes the buffering curve steeper. That is, the bicarbonate concentration increases more at greater hemoglobin concentrations as carbonic acid (in the form of CO2) is added to the blood. The increase in bicarbonate concentration is greater with more hemoglobin because as carbon dioxide is added to the blood, the hydrogen ions formed by the dissociation of carbonic acid are buffered by hemoglobin (as will be discussed shortly). Most of the bicarbonate ions formed by this dissociation can therefore move into the plasma. The buffer value of plasma in the presence of hemoglobin is thus 4 to 5 times that of plasma separated from erythrocytes. Therefore, the slope of the normal in vivo buffer line shown in Figures 8–1 and 8–3 is mainly determined by the nonbicarbonate buffers present in the body.
The phosphate buffer system mainly consists of the buffer pair of the dihydrogen phosphate (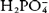 ) and the monohydrogen phosphate (
) and the monohydrogen phosphate (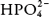 ) anions:
) anions:
The pK of the acid form is 6.8, so that in pHs ranging near 7.0, the acid form can readily donate a proton and the base form can accept a proton. Many organic phosphates found in the body also have pKs within ±0.5 pH units of 7.0, and these compounds can also function as buffers under physiologic conditions. These organic phosphates include such compounds as glucose-1-phosphate and adenosine triphosphate.
Although several potential buffering groups are found on proteins, only 1 large group has pKs in the pH range encountered in the blood. These are the imidazole groups in the histidine residues of the peptide chains. The pKs of the various histidine residues on the different plasma proteins range from about 5.5 to about 8.5, thus providing a broad spectrum of buffer pairs. The protein present in the greatest quantity in the blood is hemoglobin. Thirty-six of the 540 amino acid residues in hemoglobin are histidine, with pKs ranging from 7 to 8; the N-terminal valine residues also have a pK of about 7.8. As already noted, deoxyhemoglobin is a weaker acid than is oxyhemoglobin. That is, the pK of an imidazole group of one of the histidine residues in deoxygenated hemoglobin is greater than it is in the oxyhemoglobin state. Thus, as oxygen leaves hemoglobin in the tissue capillaries, the imidazole group removes hydrogen ions from the erythrocyte interior, allowing more carbon dioxide to be transported as bicarbonate. This process is reversed in the lungs.
The bicarbonate buffer system is the major buffer found in the interstitial fluid, including the lymph. The phosphate buffer pair is also found in the interstitial fluid. The volume of the interstitial compartment is much larger than that of the plasma, and so the interstitial fluid may play an important role in buffering.
The extracellular portion of bone contains very large deposits of calcium and phosphate salts, mainly in the form of hydroxyapatite. Although bone growth in a child causes a net production of hydrogen ions, in an otherwise healthy adult, where bone growth and resorption are in a steady state, bone salts can buffer hydrogen ions in chronic acidosis. Chronic buffering of hydrogen ions by the bone salts may therefore lead to demineralization of bone.
The intracellular proteins and organic phosphates of most cells can function to buffer both fixed acids and carbonic acid. Again, this is largely a function of the histidine groups on the proteins and phosphate groups on such compounds as ATP (adenosine triphosphate) and glucose-1-phosphate. Of course, buffering by the hemoglobin in erythrocytes is intracellular buffering.